Dolutegravir
CAS No. 1051375-16-6
Dolutegravir( GSK1349572 | GSK-1349572 | GSK 1349572 )
Catalog No. M10248 CAS No. 1051375-16-6
Dolutegravir (GSK1349572) is a potent, next-generation HIV integrase (IN) inhibitor, inhibits HIV-1 integrase-catalyzed strand transfer with IC50 of 2.7 nM.
Purity : >98% (HPLC)
 COA
COA
 Datasheet
Datasheet
 HNMR
HNMR
 HPLC
HPLC
 MSDS
MSDS
 Handing Instructions
Handing Instructions
| Size | Price / USD | Stock | Quantity |
| 2MG | 39 | In Stock |


|
| 5MG | 61 | In Stock |


|
| 10MG | 105 | In Stock |


|
| 25MG | 162 | In Stock |


|
| 50MG | 259 | In Stock |


|
| 100MG | 405 | In Stock |


|
| 200MG | 574 | In Stock |


|
| 500MG | 806 | In Stock |


|
| 1G | 1053 | In Stock |


|
Biological Information
-
Product NameDolutegravir
-
NoteResearch use only, not for human use.
-
Brief DescriptionDolutegravir (GSK1349572) is a potent, next-generation HIV integrase (IN) inhibitor, inhibits HIV-1 integrase-catalyzed strand transfer with IC50 of 2.7 nM.
-
DescriptionDolutegravir (GSK1349572) is a potent, next-generation HIV integrase (IN) inhibitor, inhibits HIV-1 integrase-catalyzed strand transfer with IC50 of 2.7 nM; inhibits HIV-1 replication in PBMCs, MT-4 cells and in the PHIV assay with EC50 of 0.51, 0.71 and 2.2 nM, respectively; demonstrates activity against site-directed molecular clones containing the raltegravir-resistant signature mutations Y143R, Q148K, N155H, and G140S/Q148H; exhibits excellent anti-HIV activity in combination with representative approved antiretroviral agents in vivo.HIV Infection Approved(In Vitro):The EC50 of Dolutegravir (S/GSK1349572) against HIV-1 is 0.51 nM in PBMCs, 0.71 nM in MT-4 cells, and 2.2 nM in the PHIV assay, which uses a pseudotyped self-inactivating virus. The 50% cytotoxic concentrations (CC50) for Dolutegravir in proliferating IM-9, U-937, MT-4, and Molt-4 cells are 4.8, 7.0, 14, and 15 μM, respectively. In unstimulated and stimulated PBMCs, the CC50 are 189 μM and 52 μM, respectively. Based on the EC50 of Dolutegravir against HIV-1 in PBMCs (i.e., 0.51 nM), this translates to a cell-based therapeutic index of at least 9,400. (In Vivo):Following a single intravenous (IV) administration of Dolutegravir, the plasma clearance is low in rats (0.23 mL/min/kg) and monkeys (2.12 mL/min/kg). The half-lives in the rat and monkey are similar, approximately 6 h, and the steady-state volume of distribution (VSS) is low. Following oral administration, Dolutegravir is rapidly absorbed with a high oral bioavailability when administered as a solution to fasted male rats and a single monkey (75.6 and 87.0%, respectively). Dolutegravir exposure (Cmax and AUC) increased with increasing dose following oral administration of a suspension to non-fasted rats up to 250 mg/kg and non-fasted monkeys up to 50 mg/kg, although the increase is less than proportional.
-
In VitroThe EC50 of Dolutegravir (S/GSK1349572) against HIV-1 is 0.51 nM in PBMCs, 0.71 nM in MT-4 cells, and 2.2 nM in the PHIV assay, which uses a pseudotyped self-inactivating virus. The 50% cytotoxic concentrations (CC50) for Dolutegravir in proliferating IM-9, U-937, MT-4, and Molt-4 cells are 4.8, 7.0, 14, and 15 μM, respectively. In unstimulated and stimulated PBMCs, the CC50 are 189 μM and 52 μM, respectively. Based on the EC50 of Dolutegravir against HIV-1 in PBMCs (i.e., 0.51 nM), this translates to a cell-based therapeutic index of at least 9,400.
-
In VivoFollowing a single intravenous (IV) administration of Dolutegravir, the plasma clearance is low in rats (0.23 mL/min/kg) and monkeys (2.12 mL/min/kg). The half-lives in the rat and monkey are similar, approximately 6 h, and the steady-state volume of distribution (VSS) is low. Following oral administration, Dolutegravir is rapidly absorbed with a high oral bioavailability when administered as a solution to fasted male rats and a single monkey (75.6 and 87.0%, respectively). Dolutegravir exposure (Cmax and AUC) increased with increasing dose following oral administration of a suspension to non-fasted rats up to 250 mg/kg and non-fasted monkeys up to 50 mg/kg, although the increase is less than proportional.
-
SynonymsGSK1349572 | GSK-1349572 | GSK 1349572
-
PathwayMicrobiology/Virology
-
TargetHIV
-
RecptorHIVintegrase
-
Research AreaInfection
-
IndicationHIV Infection
Chemical Information
-
CAS Number1051375-16-6
-
Formula Weight419.3788
-
Molecular FormulaC20H19F2N3O5
-
Purity>98% (HPLC)
-
SolubilityDMSO: 10 mg/mL
-
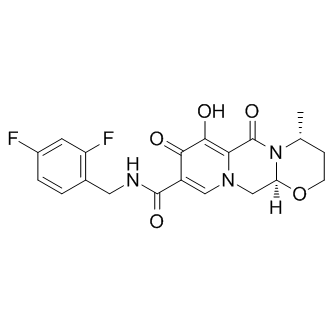
SMILES[H][C@]12CN3C=C(C(=O)NCC4=C(F)C=C(F)C=C4)C(=O)C(O)=C3C(=O)N1[C@H](C)CCO2 |r,c:10,16,22,t:4,13|
-
Chemical Name2H-Pyrido[1',2':4,5]pyrazino[2,1-b][1,3]oxazine-9-carboxamide, N-[(2,4-difluorophenyl)methyl]-3,4,6,8,12,12a-hexahydro-7-hydroxy-4-methyl-6,8-dioxo-, (4R,12aS)-
Shipping & Storage Information
-
Storage(-20℃)
-
ShippingWith Ice Pack
-
Stability≥ 2 years
Reference
1. Kobayashi M, et al. Antimicrob Agents Chemother. 2011 Feb;55(2):813-21.
2. Garrido C, et al. Antiviral Res. 2011 Jun;90(3):164-7.
3. Hightower KE, et al. Antimicrob Agents Chemother. 2011 Oct;55(10):4552-9.
4. Hare S, et al. Mol Pharmacol. 2011 Oct;80(4):565-72.
molnova catalog



related products
-
DRB
DRB is a nucleoside analog that inhibits several carboxyl-terminal domain (CTD) kinases including casein kinase II (IC50 range of 4-10 μM).
-
Saquinavir
Apotent and selective inhibitor of HIV-1 protease with IC50 of 0.5-6 nM in cell assays.
-
ABX464
ABX464 (ABX-464, ABX 464) is a novel class of anti-HIV small molecule targeting Rev-mediated viral RNA biogenesis.



 Cart
Cart
 sales@molnova.com
sales@molnova.com


