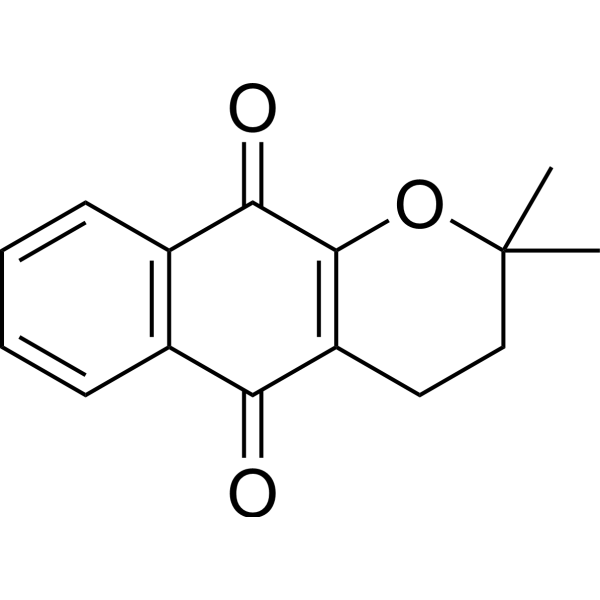
α-Lapachone
CAS No. 4707-33-9
α-Lapachone( alpha-Lapachone )
Catalog No. M29089 CAS No. 4707-33-9
alpha-Lapachone has antineoplastic activity, it shows an approximately trypanocidal activity against Trypanosoma cruzi.
Purity : >98% (HPLC)
 COA
COA
 Datasheet
Datasheet
 HNMR
HNMR
 HPLC
HPLC
 MSDS
MSDS
 Handing Instructions
Handing Instructions
| Size | Price / USD | Stock | Quantity |
| 1 mL x 10 mM in DMSO | 65 | In Stock |


|
| 5MG | 61 | In Stock |


|
| 10MG | 92 | In Stock |


|
| 25MG | 202 | In Stock |


|
| 50MG | 296 | In Stock |


|
| 100MG | 440 | In Stock |


|
| 200MG | Get Quote | In Stock |


|
| 500MG | Get Quote | In Stock |


|
| 1G | Get Quote | In Stock |


|
Biological Information
-
Product Nameα-Lapachone
-
NoteResearch use only, not for human use.
-
Brief Descriptionalpha-Lapachone has antineoplastic activity, it shows an approximately trypanocidal activity against Trypanosoma cruzi.
-
Descriptionalpha-Lapachone has antineoplastic activity, it shows an approximately trypanocidal activity against Trypanosoma cruzi.(In Vitro):The photophysical and photochemical reactions of β-lapachone were studied using femtosecond transient absorption, nanosecond transient absorption, and nanosecond time-resolved resonance Raman spectroscopy techniques and density functional theory calculations. In acetonitrile, β-lapachone underwent an efficient intersystem crossing to form the triplet state of β-lapachone. However, in water-rich solutions, the singlet state of β-lapachone was predominantly quenched by the photoinduced protonation of the carbonyl group at the β position (O9). After protonation, a series of fast reaction steps occurred to eventually generate the triplet state α±-lapachone intermediate. This triplet state of α±-lapachone then underwent intersystem crossing to produce the ground singlet state of α±-lapachone as the final product. 1,2-Naphthoquinone is examined in acetonitrile and water solutions in order to elucidate the important roles that water and the pyran ring play during the photoconversion from β-lapachone to α±-lapachone. β-Lapachone can also be converted to α±-lapachone in the ground state when a strong acid is added to an aqueous solution.
-
In Vitro——
-
In Vivo——
-
Synonymsalpha-Lapachone
-
PathwayCell Cycle/DNA Damage
-
TargetTopoisomerase
-
RecptorTopoisomerase
-
Research Area——
-
Indication——
Chemical Information
-
CAS Number4707-33-9
-
Formula Weight242.274
-
Molecular FormulaC15H14O3
-
Purity>98% (HPLC)
-
SolubilityIn Vitro:?DMSO : 100 mg/mL (412.76 mM)
-
SMILESCC1(C)CCC2=C(O1)C(=O)c1ccccc1C2=O
-
Chemical Name——
Shipping & Storage Information
-
Storage(-20℃)
-
ShippingWith Ice Pack
-
Stability≥ 2 years
Reference
molnova catalog



related products
-
Zoliflodacin
Zoliflodacin (ETX0914, AZD0914) is a new bacterial DNA gyrase/topoisomerase inhibitor. Zoliflodacin has potent in vitro antibacterial activity against Gram-positive and Gram-negative organisms, including S. aureus (MIC90: 0.25 μg/mL).
-
Indotecan
Indotecan is an effective inhibitor of topoisomerase 1(Top1).
-
DRF-1042
DRF-1042, an orally active camptothecin analog, exhibits anticancer and insecticidal activities by inhibiting DNA topoisomerase I. It has been studied for its efficacy against solid tumors, including prostate and colon cancers.



 Cart
Cart
 sales@molnova.com
sales@molnova.com


