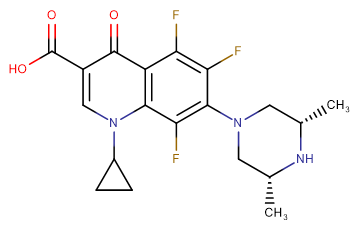
Orbifloxacin
CAS No. 113617-63-3
Orbifloxacin( CP 104,354 )
Catalog No. M10479 CAS No. 113617-63-3
Orbifloxacin is a synthetic broad-spectrum fluoroquinolone antibiotic.
Purity : >98% (HPLC)
 COA
COA
 Datasheet
Datasheet
 HNMR
HNMR
 HPLC
HPLC
 MSDS
MSDS
 Handing Instructions
Handing Instructions
| Size | Price / USD | Stock | Quantity |
| 100MG | Get Quote | In Stock |


|
| 200MG | 28 | In Stock |


|
| 500MG | Get Quote | In Stock |


|
| 1G | Get Quote | In Stock |


|
Biological Information
-
Product NameOrbifloxacin
-
NoteResearch use only, not for human use.
-
Brief DescriptionOrbifloxacin is a synthetic broad-spectrum fluoroquinolone antibiotic.
-
DescriptionOrbifloxacin is a synthetic broad-spectrum fluoroquinolone antibiotic.
-
In Vitro——
-
In Vivo——
-
SynonymsCP 104,354
-
PathwayOthers
-
TargetOther Targets
-
RecptorOthers
-
Research AreaInfection
-
Indication——
Chemical Information
-
CAS Number113617-63-3
-
Formula Weight395.38
-
Molecular FormulaC19H20F3N3O3
-
Purity>98% (HPLC)
-
SolubilityDMSO: 0.05 mg/mL (0.12 mM)
-
SMILESO=C(C1=CN(C2CC2)C3=C(C(F)=C(F)C(N4C[C@H](C)N[C@H](C)C4)=C3F)C1=O)O
-
Chemical Name1-Cyclopropyl-7-[(3S,5R)-3,5-dimethylpiperazin-1-yl]-5,6,8-trifluoro-4-oxoquinoline-3-carboxylic acid
Shipping & Storage Information
-
Storage(-20℃)
-
ShippingWith Ice Pack
-
Stability≥ 2 years
Reference
1. Ganière JP, et al. Res Vet Sci. 2004 Aug; 77(1):67-7
molnova catalog



related products
-
KPT-6566
KPT-6566 is a novel selective covalent pin1 inhibitor KPT-6566 shows an IC50 of 640?nM and a Ki of 625.2 nM for PIN1 PPIase domainand has anti-cancer activity.
-
Gingerglycolipid A
Gingerglycolipid A is a monoacyldigalactosyl glycerol.
-
CCTA-1523
CCTA-1523 is an efflux function of ABCG2 inhibitor. CCTA-1523 selectively reverses ABCG2-mediated MDR in cancer cells.



 Cart
Cart
 sales@molnova.com
sales@molnova.com


