Thapsigargin
CAS No. 67526-95-8
Thapsigargin( G-202 | G 202 | G202 )
Catalog No. M15579 CAS No. 67526-95-8
A potent, selective, non-competitive inhibitor of the sarco/endoplasmic reticulum Ca2+ ATPase (SERCA), causes ER stress and induces autophagy in mammalian cells.
Purity : >98% (HPLC)
 COA
COA
 Datasheet
Datasheet
 HNMR
HNMR
 HPLC
HPLC
 MSDS
MSDS
 Handing Instructions
Handing Instructions
| Size | Price / USD | Stock | Quantity |
| 100MG | Get Quote | In Stock |


|
| 200MG | Get Quote | In Stock |


|
| 500MG | Get Quote | In Stock |


|
| 1G | Get Quote | In Stock |


|
Biological Information
-
Product NameThapsigargin
-
NoteResearch use only, not for human use.
-
Brief DescriptionA potent, selective, non-competitive inhibitor of the sarco/endoplasmic reticulum Ca2+ ATPase (SERCA), causes ER stress and induces autophagy in mammalian cells.
-
DescriptionA potent, selective, non-competitive inhibitor of the sarco/endoplasmic reticulum Ca2+ ATPase (SERCA), causes ER stress and induces autophagy in mammalian cells; inhibits the Ca2+ ATPase of rat liver ER about 100% at 170 nM, but no inhibition for skeletal muscle SR; increases the concentration of cytosolic free Ca2+ in sensitive cells by an acute and highly specific arrest of the endoplasmic reticulum Ca2+ pump.
-
In VitroCell Proliferation Assay Cell Line:MH7A human rheumatoid arthritis synovial cells Concentration:0.001, 0.1, and 1?μM Incubation Time:For 2 and 4 days Result:Arrested cell proliferations in a time- and dose-dependent manner.Apoptosis Analysis Cell Line:MH7A human rheumatoid arthritis synovial cells Concentration:0.001, 0.1, and 1?μM Incubation Time:For 2 and 4 days Result:Induces cell apoptosis in a time- and dose-dependent manner.Western Blot Analysis Cell Line:MH7A human rheumatoid arthritis synovial cells Concentration:0.001, 0.1, and 1?μM Incubation Time:For 2 and 4 days Result:Impairs mTOR activity and leads to cyclin D1 expressions
-
In VivoAnimal Model:Male Balb/c mice (20-25 g) Dosage:0.25 ug/g, 0.5 ug/g and 1 ug/g Administration:Injection; 24 hours Result:Increased of 2 to 5-fold in chemokine and pro-inflammatory expression.
-
SynonymsG-202 | G 202 | G202
-
PathwayAutophagy
-
TargetAutophagy
-
RecptorAutophagy
-
Research AreaCancer
-
Indication——
Chemical Information
-
CAS Number67526-95-8
-
Formula Weight650.76
-
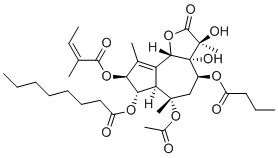
Molecular FormulaC34H50O12
-
Purity>98% (HPLC)
-
SolubilityIn Vitro:?DMSO : 50 mg/mL (76.83 mM)
-
SMILESCCCCCCCC(O[C@@H]1[C@@H](OC(/C(C)=C\C)=O)C(C)=C2[C@@]1([H])[C@@](C)(OC(C)=O)C[C@H](OC(CCC)=O)[C@@]([C@@]3(O)C)(O)[C@@]2([H])OC3=O)=O
-
Chemical Name(3S,3aR,4S,6S,6AR,7S,8S,9bS)-6- (Acetyloxy)-2,3,3a,4,5,6,6a,7,8,9b-decahydro-3,3a-dihydroxy-3,6,9-trimethyl-8-[[(2Z)-2-methyl-1-oxo-2-butenyl]oxy]-2-oxo-4-(1-oxobutoxy)azuleno[4,5-b]furan-7-yl octanoate
Shipping & Storage Information
-
Storage(-20℃)
-
ShippingWith Ice Pack
-
Stability≥ 2 years
Reference
1. Davidson GA, et al. J Biol Chem. 1995 May 19;270(20):11731-4.
2. Thastrup O, et al. Proc Natl Acad Sci U S A. 1990 Apr;87(7):2466-70.
3. Yu M, et al. J Biol Chem. 1998 Feb 6;273(6):3542-6.
4. Panagaki T, et al. Sci Rep. 2017 Nov 23;7(1):16158.
molnova catalog



related products
-
Sofalcone
Sofalcone a gastric antiulcer agent in clinical use is known to induce the expression of Heme oxygenase-1 (HO-1) in gastric epithelium.
-
LC3-mHTT-IN-AN1
LC3-mHTT-IN-AN1 (Compound AN1) is a mHTT-LC3 linker compound, which interacts with both mutant huntingtin protein (mHTT) and LC3B but not with wtHTT or irrelevant control proteins.
-
Ginkgolide K
Ginkgolide K induces protective autophagy through the AMPK/mTOR/ULK1 signalling pathway. It possesses neuroprotective activity.



 Cart
Cart
 sales@molnova.com
sales@molnova.com


