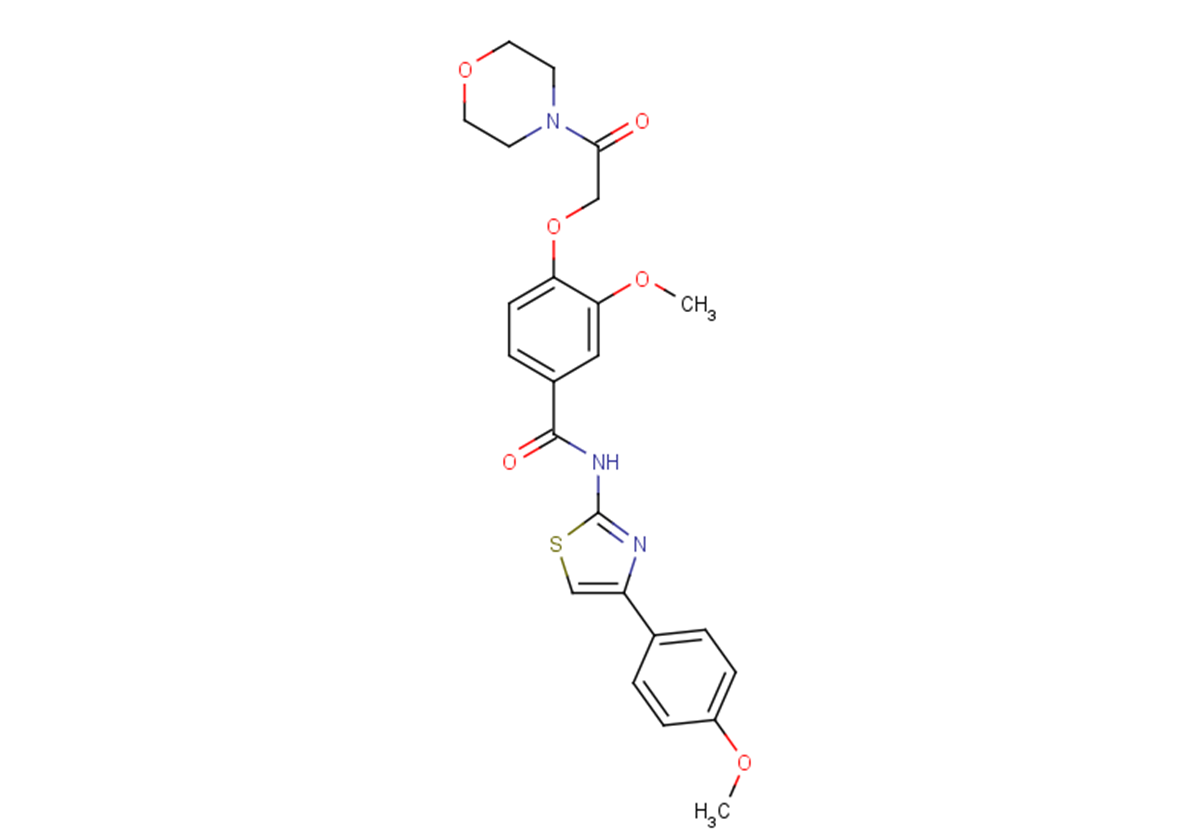
SBI-477
CAS No. 781628-99-7
SBI-477( —— )
Catalog No. M22929 CAS No. 781628-99-7
SBI-477 is a chemical probe stimulated insulin signaling by deactivating the transcription factor MondoA. SBI-477 coordinately inhibits triacylglyceride (TAG) synthesis and enhances basal glucose uptake in human skeletal myocytes.
Purity : >98% (HPLC)
 COA
COA
 Datasheet
Datasheet
 HNMR
HNMR
 HPLC
HPLC
 MSDS
MSDS
 Handing Instructions
Handing Instructions
| Size | Price / USD | Stock | Quantity |
| 5MG | 357 | In Stock |


|
| 10MG | 530 | In Stock |


|
| 25MG | 851 | In Stock |


|
| 50MG | 1152 | In Stock |


|
| 100MG | 1557 | In Stock |


|
| 500MG | 3114 | In Stock |


|
| 1G | Get Quote | In Stock |


|
Biological Information
-
Product NameSBI-477
-
NoteResearch use only, not for human use.
-
Brief DescriptionSBI-477 is a chemical probe stimulated insulin signaling by deactivating the transcription factor MondoA. SBI-477 coordinately inhibits triacylglyceride (TAG) synthesis and enhances basal glucose uptake in human skeletal myocytes.
-
DescriptionSBI-477 is a chemical probe stimulated insulin signaling by deactivating the transcription factor MondoA. SBI-477 coordinately inhibits triacylglyceride (TAG) synthesis and enhances basal glucose uptake in human skeletal myocytes. SBI-477, that coordinately inhibited triacylglyceride (TAG) synthesis and enhanced basal glucose uptake in human skeletal myocytes. SBI-477 stimulated insulin signaling by deactivating the transcription factor MondoA, leading to reduced expression of the insulin pathway suppressors thioredoxin-interacting protein (TXNIP) and arrestin domain-containing 4 (ARRDC4). Depleting MondoA in myocytes reproduced the effects of SBI-477 on glucose uptake and myocyte lipid accumulation.
-
In Vitro——
-
In Vivo——
-
Synonyms——
-
PathwayOthers
-
TargetOther Targets
-
RecptorOthers
-
Research Area——
-
Indication——
Chemical Information
-
CAS Number781628-99-7
-
Formula Weight483.54
-
Molecular FormulaC24H25N3O6S
-
Purity>98% (HPLC)
-
SolubilityDMSO:65 mg/mL (134.43 mM; Need ultrasonic)
-
SMILESO=C(NC1=NC(C2=CC=C(OC)C=C2)=CS1)C3=CC=C(OCC(N4CCOCC4)=O)C(OC)=C3
-
Chemical Name——
Shipping & Storage Information
-
Storage(-20℃)
-
ShippingWith Ice Pack
-
Stability≥ 2 years
Reference
1.Ahn B, et al. MondoA coordinately regulates skeletal myocyte lipid homeostasis and insulin signaling. J Clin Invest. 2016 Sep 1;126(9):3567-79.
molnova catalog



related products
-
Soyasaponin Aa
Soyasaponin Aa and soyasaponin Ab dose-dependently markedly inhibit adipocyte differentiation and expression of various adipogenic marker genes, through the downregulation of the adipogenesis-related transcription factors PPARγ and C/EBPα± in 3T3-L1 adipocytes.
-
A-L-A
A-L-A
-
[bAla8]-Neurokinin A...
[bAla8]-Neurokinin A(4-10) is a neurokinin 2 (NK2) receptor agonist.



 Cart
Cart
 sales@molnova.com
sales@molnova.com


