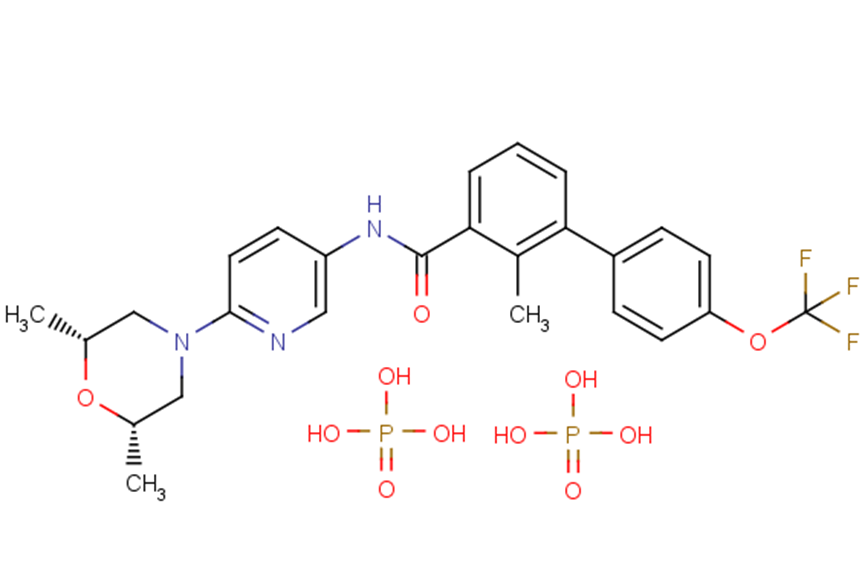
Erismodegib diphosphate
CAS No. 1218778-77-8
Erismodegib diphosphate( Sonidegib diphosphate,LDE225 diphosphate,NVP-LDE 225 diphosphate )
Catalog No. M22688 CAS No. 1218778-77-8
Erismodegib diphosphate is an effective and selective Smo antagonist (IC50: 1.3 nM and 2.5 nM for mouse and human Smo in a binding assay, respectively).
Purity : >98% (HPLC)
 COA
COA
 Datasheet
Datasheet
 HNMR
HNMR
 HPLC
HPLC
 MSDS
MSDS
 Handing Instructions
Handing Instructions
| Size | Price / USD | Stock | Quantity |
| 1 mL x 10 mM in DMSO | 63 | In Stock |


|
| 5MG | 41 | In Stock |


|
| 10MG | 49 | In Stock |


|
| 25MG | 79 | In Stock |


|
| 50MG | 108 | In Stock |


|
| 100MG | 184 | In Stock |


|
| 200MG | Get Quote | In Stock |


|
| 500MG | Get Quote | In Stock |


|
| 1G | Get Quote | In Stock |


|
Biological Information
-
Product NameErismodegib diphosphate
-
NoteResearch use only, not for human use.
-
Brief DescriptionErismodegib diphosphate is an effective and selective Smo antagonist (IC50: 1.3 nM and 2.5 nM for mouse and human Smo in a binding assay, respectively).
-
DescriptionErismodegib diphosphate is an effective and selective Smo antagonist (IC50: 1.3 nM and 2.5 nM for mouse and human Smo in a binding assay, respectively). Erismodegib diphosphate is used alone and in combination with Nilotinib, inhibits the Hh pathway in CD34+ chronic phase (CP)-chronic myeloid leukemia (CML) cells, reducing the number and self-renewal capacity of CML leukemia stem cell (LSC). In a similar fashion to cyclopamine, Erismodegib interacts directly with SMO, to reduce the expression of downstream Hh signaling targets. The IC50 values for Erismodegib diphosphate for the major human CYP450 drug-metabolizing enzymes are greater than 10 μM. Primary CD34+ CP-CML cells are cultured in serum-free media (SFM)±Erismodegib for 6, 24, and 72 hours (h). At 72 h, while there is variability between the biological samples, GLI1 is obviously downregulated following exposure to Erismodegib (10 nM; 0.78-fold and 100 nM; 0.73-fold, respectively (p<0.01).Erismodegib diphosphate is a weak base with a measured pKa of 4.2 and exhibits relatively poor aqueous solubility. Erismodegib diphosphate demonstrates dose-related antitumor activity after 10 days of oral administration of a suspension of the diphosphate salt, in the subcutaneous Ptch+/-p53-/- medulloblastoma allograft mouse model. Bone marrow cells and spleen cells from a subset of treated mice are transplanted into secondary recipient mice. Erismodegib diphosphate (5 mg/kg/day; once daily) obviously inhibits tumor growth, corresponding to a T/C value of 33% (p<0.05 as compared to vehicle controls). Erismodegib affords 51 and 83% regression respectively when dosed at 10 and 20 mg/kg/day qd. Transplantation of either bone marrow (BM) or spleen cells from mice treated with Erismodegib diphosphate+ Nilotinib results in reduced white cell count (WCC) and reduces leukemia development in secondary recipients compared to Erismodegib or Nilotinib alone.(In Vitro):The IC50 values for Sonidegib (NVP-LDE225) for the major human CYP450 drug metabolizing enzymes is greater than 10 μM. Sonidegib (LDE225), a small molecule, clinically investigated SMO inhibitor, used alone and in combination with Nilotinib, inhibits the Hh pathway in CD34+ chronic phase (CP)-chronic myeloid leukaemia (CML) cells, reducing the number and self-renewal capacity of CML leukaemia stem cell (LSC). Sonidegib interacts directly with SMO, in a similar fashion to cyclopamine, to reduce expression of downstream Hh signaling targets. Primary CD34+ CP-CML cells are cultured in serum free media (SFM)±Sonidegib for 6, 24 and 72 hours (h). At 72 h, while there is variability between the biological samples, GLI1 is significantly downregulated following exposure to Sonidegib (10 nM; 0.78-fold and 100 nM; 0.73-fold, respectively (p<0.01). (In Vivo):Sonidegib (NVP-LDE225) is a weak base with a measured pKa of 4.2 and exhibits relatively poor aqueous solubility. In the subcutaneous Ptch+/-p53-/- medulloblastoma allograft mouse model, Sonidegib demonstrates dose-related antitumor activity after 10 days of oral administration of a suspension of the diphosphate salt. At a dose of 5 mg/kg/day qd, Sonidegib significantly inhibits tumor growth, corresponding to a T/C value of 33% (p<0.05 as compared to vehicle controls). When dosed at 10 and 20 mg/kg/day qd, Sonidegib affords 51 and 83% regression, respectively. Bone marrow cells and spleen cells from a subset of treated mice are transplanted into secondary recipient mice. Transplantation of either bone marrow (BM) or spleen cells from mice treated with Sonidegib (LDE225)+Nilotinib results in reduced white cell count (WCC) and reduces leukaemia development in secondary recipients compared to Sonidegib or Nilotinib alone.
-
In VitroThe IC50 values for Sonidegib (NVP-LDE225) for the major human CYP450 drug metabolizing enzymes is greater than 10 μM. Sonidegib (LDE225), a small molecule, clinically investigated SMO inhibitor, used alone and in combination with Nilotinib, inhibits the Hh pathway in CD34+ chronic phase (CP)-chronic myeloid leukaemia (CML) cells, reducing the number and self-renewal capacity of CML leukaemia stem cell (LSC). Sonidegib interacts directly with SMO, in a similar fashion to cyclopamine, to reduce expression of downstream Hh signaling targets. Primary CD34+ CP-CML cells are cultured in serum free media (SFM)±Sonidegib for 6, 24 and 72 hours (h). At 72 h, while there is variability between the biological samples, GLI1 is significantly downregulated following exposure to Sonidegib (10 nM; 0.78-fold and 100 nM; 0.73-fold, respectively (p<0.01).
-
In VivoSonidegib (NVP-LDE225) is a weak base with a measured pKa of 4.2 and exhibits relatively poor aqueous solubility. In the subcutaneous Ptch+/-p53-/- medulloblastoma allograft mouse model, Sonidegib demonstrates dose-related antitumor activity after 10 days of oral administration of a suspension of the diphosphate salt. At a dose of 5 mg/kg/day qd, Sonidegib significantly inhibits tumor growth, corresponding to a T/C value of 33% (p<0.05 as compared to vehicle controls). When dosed at 10 and 20 mg/kg/day qd, Sonidegib affords 51 and 83% regression, respectively. Bone marrow cells and spleen cells from a subset of treated mice are transplanted into secondary recipient mice. Transplantation of either bone marrow (BM) or spleen cells from mice treated with Sonidegib (LDE225)+Nilotinib results in reduced white cell count (WCC) and reduces leukaemia development in secondary recipients compared to Sonidegib or Nilotinib alone.
-
SynonymsSonidegib diphosphate,LDE225 diphosphate,NVP-LDE 225 diphosphate
-
PathwayWnt/Notch/Hedgehog
-
TargetSmoothened (Smo)
-
RecptormSmo|hSmo
-
Research AreaCancer
-
IndicationBasal Cell Carcinoma|Gorlin Syndrome|Nevoid Basal Cell Carcinoma Syndrome
Chemical Information
-
CAS Number1218778-77-8
-
Formula Weight681.49
-
Molecular FormulaC26H32F3N3O11P2
-
Purity>98% (HPLC)
-
SolubilityDMSO:99 mg/mL(145.27 mM);H2O:0.25 mg/mL (0.37 mM; Need ultrasonic)
-
SMILESOP(O)(O)=O.OP(O)(O)=O.C[C@H]1CN(C[C@@H](C)O1)c1ccc(NC(=O)c2cccc(-c3ccc(OC(F)(F)F)cc3)c2C)cn1
-
Chemical Name——
Shipping & Storage Information
-
Storage(-20℃)
-
ShippingWith Ice Pack
-
Stability≥ 2 years
Reference
1. Pan S, et al. Discovery of NVP-LDE225, a Potent and Selective Smoothened Antagonist. ACS Med Chem Lett. 2010 Mar 16;1(3):130-4.
molnova catalog



related products
-
PF-5274857 freebase
PF-5274857 is an effective and selective hedgehog signaling pathway inhibitor (IC50: 5.8 nM and a Ki: 4.6 nM).
-
IHR-1
IHR-1 is a Potent Smo antagonist (IC50 = 7.6 nM). Selectively inhibits Hedgehog signaling over Wnt and Notch signaling pathways. Blocks Smo accumulation in primary cilium in vitro.
-
GI-560192
GI-560192 (RL-0070933) is a potent smo cilial modulator with an EC50 value of 0.02 μM. GI-560192 modulates the translocation and/or accumulation of smoothened to the primary cilia by hedgehog signaling pathway.



 Cart
Cart
 sales@molnova.com
sales@molnova.com


