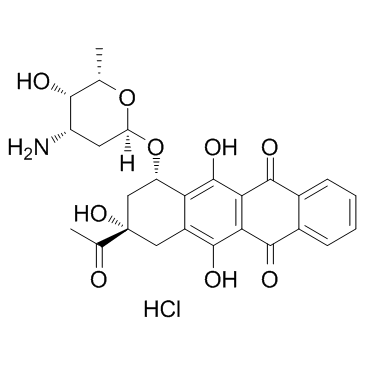
Idarubicin hydrochloride
CAS No. 57852-57-0
Idarubicin hydrochloride( 4-Demethoxydaunorubicin hydrochloride )
Catalog No. M15119 CAS No. 57852-57-0
An anthracycline antileukemic that inserts into DNA and prevents DNA unwinding by interfering with the enzyme Topoisomerase II in cancer cells.
Purity : >98% (HPLC)
 COA
COA
 Datasheet
Datasheet
 HNMR
HNMR
 HPLC
HPLC
 MSDS
MSDS
 Handing Instructions
Handing Instructions
| Size | Price / USD | Stock | Quantity |
| 1 mL x 10 mM in DMSO | 64 | In Stock |


|
| 5MG | 52 | In Stock |


|
| 10MG | 92 | In Stock |


|
| 25MG | 155 | In Stock |


|
| 100MG | Get Quote | In Stock |


|
| 200MG | Get Quote | In Stock |


|
| 500MG | Get Quote | In Stock |


|
| 1G | Get Quote | In Stock |


|
Biological Information
-
Product NameIdarubicin hydrochloride
-
NoteResearch use only, not for human use.
-
Brief DescriptionAn anthracycline antileukemic that inserts into DNA and prevents DNA unwinding by interfering with the enzyme Topoisomerase II in cancer cells.
-
DescriptionAn anthracycline antileukemic that inserts into DNA and prevents DNA unwinding by interfering with the enzyme Topoisomerase II in cancer cells; inhibits the proliferation of MCF-7 cells with IC50 of 0.1 uM; also induces histone eviction from chromatin.Chemotherapeutic Agents Approved(In Vitro):The IC50 of idarubicin is 3.3±0.4 ng/mL on MCF-7 monolayers and 7.9±1.1 ng/mL in multicellular spheroids. Idarubicin has shown a greater cytotoxicity than daunorubicin or doxorubicin in various in vitro systems. This has been attributed to a better ability of idarubicin to induce the formation of topoisomerase II -mediated DNA breaks.Idarubicin is about 57.5-fold and 25-fold more active than doxorubicin and epirubicin, respectively. Idarubicin produces a concentration-dependent reduction in cell growth, with an IC50 value of approximately 0.01 μM. Idarubicin produced a concentration-dependent inhibition of DNA synthesis.
-
In VitroThe IC50 of Idarubicin hydrochloride is 3.3±0.4 ng/mL on MCF-7 monolayers and 7.9±1.1 ng/mL in multicellular spheroids. Idarubicin hydrochloride has shown a greater cytotoxicity than daunorubicin or doxorubicin in various in vitro systems. This has been attributed to a better ability of idarubicin to induce the formation of topoisomerase II -mediated DNA breaks. Idarubicin hydrochloride is about 57.5-fold and 25-fold more active than doxorubicin and epirubicin, respectively. Idarubicin hydrochloride produces a concentration-dependent reduction in cell growth, with an IC50 value of approximately 0.01 μM. Idarubicin hydrochloride produced a concentration-dependent inhibition of DNA synthesis.
-
In Vivo——
-
Synonyms4-Demethoxydaunorubicin hydrochloride
-
PathwayCell Cycle/DNA Damage
-
TargetTopoisomerase
-
RecptorMulticellular spheroids| Topo II (MCF-7 cells)
-
Research AreaCancer
-
IndicationChemotherapeutic
Chemical Information
-
CAS Number57852-57-0
-
Formula Weight533.9548
-
Molecular FormulaC26H28ClNO9
-
Purity>98% (HPLC)
-
Solubility10 mM in DMSO
-
SMILESO=C1C2=C(O)C([C@@H](O[C@H]3C[C@H](N)[C@H](O)[C@H](C)O3)C[C@](O)(C(C)=O)C4)=C4C(O)=C2C(C5=C1C=CC=C5)=O.[H]Cl
-
Chemical Name5,12-Naphthacenedione, 9-acetyl-7-[(3-amino-2,3,6-trideoxy-α-L-lyxo-hexopyranosyl)oxy]-7,8,9,10-tetrahydro-6,9,11-trihydroxy-, hydrochloride (1:1), (7S,9S)-
Shipping & Storage Information
-
Storage(-20℃)
-
ShippingWith Ice Pack
-
Stability≥ 2 years
Reference
1. Gewirtz DA, et al. Cancer Chemother Pharmacol. 1998;41(5):361-9.
2. Pang B, et al. Nat Commun. 2013;4:1908.
3. Nitiss JL. Nat Rev Cancer. 2009 May;9(5):338-50.
molnova catalog



related products
-
ARN-21934
ARN-21934 is a potent and selective inhibitor for human topoisomerase II α and topoisomerase II β. ARN-21934(IC50 = 2 μM) inhibits DNA relaxation as compared to the anticancer agent Etoposide (IC50=120 μM).
-
Betulinic acid
A naturally occurring pentacyclic triterpenoid which has antiretroviral, antimalarial, and anti-inflammatory properties.
-
Irinotecan Hydrochlo...
Irinotecan Hcl(CPT-11 Hcl) prevents DNA from unwinding by inhibition of topoisomerase 1.



 Cart
Cart
 sales@molnova.com
sales@molnova.com


