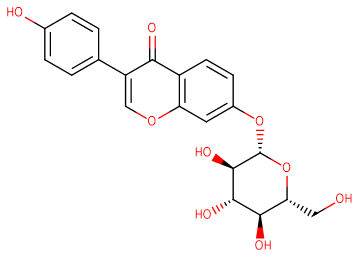
Daidzin
CAS No. 552-66-9
Daidzin( Daidzoside | NPI 031D )
Catalog No. M15003 CAS No. 552-66-9
Daidzin is an isoflavone that has anti-oxidant, anti-carcinogenic, and anti-atherosclerotic activities.
Purity : >98% (HPLC)
 COA
COA
 Datasheet
Datasheet
 HNMR
HNMR
 HPLC
HPLC
 MSDS
MSDS
 Handing Instructions
Handing Instructions
| Size | Price / USD | Stock | Quantity |
| 50MG | 59 | In Stock |


|
| 100MG | Get Quote | In Stock |


|
| 200MG | Get Quote | In Stock |


|
| 500MG | Get Quote | In Stock |


|
| 1G | Get Quote | In Stock |


|
Biological Information
-
Product NameDaidzin
-
NoteResearch use only, not for human use.
-
Brief DescriptionDaidzin is an isoflavone that has anti-oxidant, anti-carcinogenic, and anti-atherosclerotic activities.
-
DescriptionDaidzin is an isoflavone that has anti-oxidant, anti-carcinogenic, and anti-atherosclerotic activities; directly inhibits mitochondrial aldehyde dehydrogenase 2 (IC50 = 80 nM) and is an effective anti-dipsotropic isoflavone.
-
In Vitro——
-
In Vivo——
-
SynonymsDaidzoside | NPI 031D
-
PathwayMetabolic Enzyme/Protease
-
TargetDehydrogenase
-
RecptorALDH-Ⅰ| ALDH-Ⅱ
-
Research AreaOther Indications
-
Indication——
Chemical Information
-
CAS Number552-66-9
-
Formula Weight416.37
-
Molecular FormulaC21H20O9
-
Purity>98% (HPLC)
-
SolubilityDMSO: 10 mM
-
SMILESO=C1C(C2=CC=C(O)C=C2)=COC3=C1C=CC(O[C@@H]4O[C@H](CO)[C@@H](O)[C@H](O)[C@H]4O)=C3
-
Chemical Name3-(4-Hydroxyphenyl)-7-[(2S,3R,4S,5S,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]oxychromen-4-one
Shipping & Storage Information
-
Storage(-20℃)
-
ShippingWith Ice Pack
-
Stability≥ 2 years
Reference
1.Zaheer M, Reddy VD, Giri CC. Nat Prod Res. 2015 Jul 8:1-6.
molnova catalog



related products
-
Enoxolone
Enoximone is a selective phosphodiesterase inhibitor with vasodilating and positive inotropic activity that does not cause changes in myocardial oxygen consumption.
-
Epostane
Epostane acts as an antiprogestogen and terminates pregnancy by inhibiting 3β-hydroxysteroid dehydrogenase and preventing the biosynthesis of progesterone and pregnenolone.
-
PHGDH-IN-3
PHGDH-IN-3 is an orally active phosphoglycerate dehydrogenase (PHGDH) inhibitor with an IC50 of 2.8 μM against PHGDH, and it can be used in cancer research.



 Cart
Cart
 sales@molnova.com
sales@molnova.com


