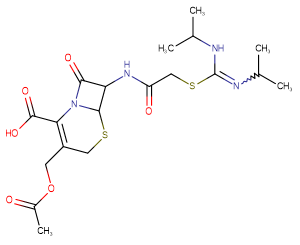
Cefathiamidine
CAS No. 33075-00-2
Cefathiamidine( —— )
Catalog No. M21185 CAS No. 33075-00-2
Cefathiamidine??exhibits a wide spectrum of antimicrobial activity against bacteria.?Cefathiamidine is used for the treatment of respiratory liver five senses urinary tract infections endocarditis and sepsis.
Purity : >98% (HPLC)
 COA
COA
 Datasheet
Datasheet
 HNMR
HNMR
 HPLC
HPLC
 MSDS
MSDS
 Handing Instructions
Handing Instructions
| Size | Price / USD | Stock | Quantity |
| 10MG | 35 | Get Quote |


|
| 25MG | 61 | Get Quote |


|
| 50MG | 95 | Get Quote |


|
| 100MG | 141 | Get Quote |


|
| 200MG | Get Quote | Get Quote |


|
| 500MG | Get Quote | Get Quote |


|
| 1G | Get Quote | Get Quote |


|
Biological Information
-
Product NameCefathiamidine
-
NoteResearch use only, not for human use.
-
Brief DescriptionCefathiamidine??exhibits a wide spectrum of antimicrobial activity against bacteria.?Cefathiamidine is used for the treatment of respiratory liver five senses urinary tract infections endocarditis and sepsis.
-
DescriptionCefathiamidine??exhibits a wide spectrum of antimicrobial activity against bacteria.?Cefathiamidine is used for the treatment of respiratory liver five senses urinary tract infections endocarditis and sepsis.(In Vitro):The in-vitro activity of Cefathiamidine against Streptococcus faecalis and Streptococcus faecium are studied in comparison with other β-lactams. All the 56 strains of Str. faecalis tested are inhibited by 2 mg/L of Cefathiamidine. The MBCs of Cefathiamidine and Ampicillin for ten strains of Str. faecalis show that the ratios of MBC/MIC are greater than 64. The rates of killing of Str. faecalis are reduced at concentrations of Cefathiamidine and Ampicillin greater than the MIC. The most rapid killing is obtained at 2 mg/L Cefathiamidine or 4 mg/L of Ampicillin. With the addition of 1 mg/L gentamicin this paradoxical bacteriocidal effect is eliminated. Time killing studies show99.9% of the cells are killed within 6 hours by a combination of aminoglycoside and β-lactam.(In Vivo):Cefathiamidine is not absorbed orally and is, thus, administered through the parenteral route (intravenously or intramuscularly). Cefathiamidine is widely distributed in most bodily fluids and tissues; however, Cefathiamidine cannot pass through the blood-brain barrier. The protein-binding capacity of Cefathiamidine is 23%, and more than 90% of Cefathiamidine is excreted unchanged by the kidney.
-
In VitroThe in-vitro activity of Cefathiamidine against Streptococcus faecalis and Streptococcus faecium are studied in comparison with other β-lactams. All the 56 strains of Str. faecalis tested are inhibited by 2 mg/L of Cefathiamidine. The MBCs of Cefathiamidine and Ampicillin for ten strains of Str. faecalis show that the ratios of MBC/MIC are greater than 64. The rates of killing of Str. faecalis are reduced at concentrations of Cefathiamidine and Ampicillin greater than the MIC. The most rapid killing is obtained at 2 mg/L Cefathiamidine or 4 mg/L of Ampicillin. With the addition of 1 mg/L gentamicin this paradoxical bacteriocidal effect is eliminated. Time killing studies show99.9% of the cells are killed within 6 hours by a combination of aminoglycoside and β-lactam.
-
In VivoCefathiamidine is not absorbed orally and is, thus, administered through the parenteral route (intravenously or intramuscularly). Cefathiamidine is widely distributed in most bodily fluids and tissues; however, Cefathiamidine cannot pass through the blood-brain barrier. The protein-binding capacity of Cefathiamidine is 23%, and more than 90% of Cefathiamidine is excreted unchanged by the kidney.
-
Synonyms——
-
PathwayGPCR/G Protein
-
TargetAntibacterial
-
RecptorBacterial
-
Research AreaInflammation/Immunology
-
IndicationRespiratory tract infection biliary tract infection urinary tract infection gynecological infection
Chemical Information
-
CAS Number33075-00-2
-
Formula Weight472.6
-
Molecular FormulaC19H28N4O6S2
-
Purity>98% (HPLC)
-
SolubilityDMSO:250 mg/mL (529.01 mM; Need ultrasonic)
-
SMILESCC(=O)OCC1=C(C(=O)O)N2C(=O)C(NC(=O)CSC(=NC(C)C)NC(C)C)C2SC1
-
Chemical Name——
Shipping & Storage Information
-
Storage(-20℃)
-
ShippingWith Ice Pack
-
Stability≥ 2 years
Reference
1 .Chen HY et al. The killing effects of cefathiamidine or ampicillin alone and in combination with gentamicin against enterococci. J Antimicrob Chemother. 1983 Jul;12(1):19-26.
molnova catalog



related products
-
Sulfaproxiline
Sulfaproxiline is a synthetic antibacterial drug of sulfonamide.
-
NITD-564
NITD-564 is a novel, direct inhibitor of the mycobacterial enoyl-reductase InhA with IC50 of 0.59 uM, exhibits MIC50 of 160 nM against Mtb.
-
Ombuin
Ombuin has anti-inflammatory activity, it inhibits some macrophage functions involved in the inflammatory process.



 Cart
Cart
 sales@molnova.com
sales@molnova.com


