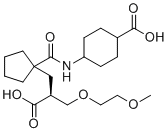
Candoxatrilat
CAS No. 123122-54-3
Candoxatrilat( UK 73967 )
Catalog No. M10911 CAS No. 123122-54-3
A potent neutral endopeptidase EC 3.4.24.11 (atriopeptidase) inhibitor.
Purity : >98% (HPLC)
 COA
COA
 Datasheet
Datasheet
 HNMR
HNMR
 HPLC
HPLC
 MSDS
MSDS
 Handing Instructions
Handing Instructions
| Size | Price / USD | Stock | Quantity |
| 100MG | Get Quote | Get Quote |


|
| 200MG | Get Quote | Get Quote |


|
| 500MG | Get Quote | Get Quote |


|
| 1G | Get Quote | Get Quote |


|
Biological Information
-
Product NameCandoxatrilat
-
NoteResearch use only, not for human use.
-
Brief DescriptionA potent neutral endopeptidase EC 3.4.24.11 (atriopeptidase) inhibitor.
-
DescriptionA potent neutral endopeptidase EC 3.4.24.11 (atriopeptidase) inhibitor that increases endogenous ANF levels and produces natriuretic and diuretic responses intravenously in mice.Heart Failure Phase 1 Discontinued.
-
In Vitro——
-
In Vivo——
-
SynonymsUK 73967
-
PathwayMetabolic Enzyme/Protease
-
TargetNeprilysin
-
RecptorNeprilysin
-
Research Area——
-
Indication——
Chemical Information
-
CAS Number123122-54-3
-
Formula Weight399.484
-
Molecular FormulaC20H33NO7
-
Purity>98% (HPLC)
-
Solubility——
-
SMILESCOCCOC[C@@H](C(=O)O)CC1(CCCC1)C(=O)N[C@H]2CC[C@H](CC2)C(=O)O
-
Chemical Name4-[[1-[(2S)-2-carboxy-3-(2-methoxyethoxy)propyl]cyclopentanecarbonyl]amino]cyclohexane-1-carboxylic acid
Shipping & Storage Information
-
Storage(-20℃)
-
ShippingWith Ice Pack
-
Stability≥ 2 years
Reference
1. Danilewicz JC, et al. Biochem Biophys Res Commun. 1989 Oct 16;164(1):58-65.
2. Shepperson NB, et al. Br J Pharmacol. 1989 Dec;98 Suppl:822P.
3. Jardine AG, et al. Am J Hypertens. 1990 Sep;3(9):661-7.
4. Northridge DB, et al. Lancet. 1989 Sep 9;2(8663):591-3.
molnova catalog



related products
-
Candoxatrilat
A potent neutral endopeptidase EC 3.4.24.11 (atriopeptidase) inhibitor.
-
Candoxatril
The orally active prodrug of candoxatrilat (UK-73967), potent neutral endopeptidase EC 3.4.24.11 (atriopeptidase) inhibitor.
-
Sacubitrilat
Sacubitrilat (LBQ657) is an effective inhibitor of active neprilysin (NEP).Sacubitrilat is bound to the active site of NEP by an intricate network of interactions that involves all functional groups of the compound giving rise to the high inhibitory potency of 5?nM.



 Cart
Cart
 sales@molnova.com
sales@molnova.com


