β2AR-IN-15
CAS No. 2088234-50-6
β2AR-IN-15( —— )
Catalog No. M13255 CAS No. 2088234-50-6
An allosteric, small-molecule negative modulator for β2AR with Kd of 1.7 uM.
Purity : >98% (HPLC)
 COA
COA
 Datasheet
Datasheet
 HNMR
HNMR
 HPLC
HPLC
 MSDS
MSDS
 Handing Instructions
Handing Instructions
| Size | Price / USD | Stock | Quantity |
| 100MG | Get Quote | Get Quote |


|
| 200MG | Get Quote | Get Quote |


|
| 500MG | Get Quote | Get Quote |


|
| 1G | Get Quote | Get Quote |


|
Biological Information
-
Product Nameβ2AR-IN-15
-
NoteResearch use only, not for human use.
-
Brief DescriptionAn allosteric, small-molecule negative modulator for β2AR with Kd of 1.7 uM.
-
DescriptionAn allosteric, small-molecule negative modulator for β2AR with Kd of 1.7 uM; binds in proximity to the G-protein binding site on the cytosolic surface of the β2AR; inhibits cAMP production through the β2AR, but not that mediated by other Gs-coupled receptors; also similarly inhibits β-arrestin recruitment to the activated β2AR.
-
In Vitro——
-
In Vivo——
-
Synonyms——
-
PathwayAngiogenesis
-
TargetAdrenergic Receptor
-
RecptorAdrenergic Receptor
-
Research Area——
-
Indication——
Chemical Information
-
CAS Number2088234-50-6
-
Formula Weight647.614
-
Molecular FormulaC34H39BrN4O4
-
Purity>98% (HPLC)
-
Solubility——
-
SMILES——
-
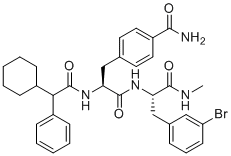
Chemical Name4-((2S)-3-(((S)-3-(3-bromophenyl)-1-(methylamino)-1-oxopropan-2-yl)amino)-2-(2-cyclohexyl-2-phenylacetamido)-3-oxopropyl)benzamide
Shipping & Storage Information
-
Storage(-20℃)
-
ShippingWith Ice Pack
-
Stability≥ 2 years
Reference
1. Ahn S, et al. Proc Natl Acad Sci U S A. 2017 Feb 14;114(7):1708-1713.
molnova catalog



related products
-
Periplogenin
Periplogenin plays protective roles against thyrotoxicosis and associated cardiovascular problems, are mediated through its direct antithyroidal and/or LPO inhibiting properties.
-
AGN192836
AGN192836 (AGN 192836) is a potent, selective α2 adrenergic (alpha 2-adrenoceptor) agonist with EC50 of 8.7, 41 and 6.6 nM for α2A, α2B and α2C receptor, respectively.
-
Dexmedetomidine hydr...
An agonist of α2-adrenergic receptor that used in veterinary medicine for its analgesic and sedative properties.



 Cart
Cart
 sales@molnova.com
sales@molnova.com


