Treosulfan
CAS No. 299-75-2
Treosulfan( NSC 39069 | Treosulphan )
Catalog No. M24222 CAS No. 299-75-2
Treosulfan is a bifunctional alkylating agent. It also has activity in ovarian cancer and other solid tumor types.
Purity : >98% (HPLC)
 COA
COA
 Datasheet
Datasheet
 HNMR
HNMR
 HPLC
HPLC
 MSDS
MSDS
 Handing Instructions
Handing Instructions
| Size | Price / USD | Stock | Quantity |
| 2MG | 41 | In Stock |


|
| 5MG | 68 | In Stock |


|
| 10MG | 113 | In Stock |


|
| 25MG | 232 | In Stock |


|
| 50MG | 438 | In Stock |


|
| 100MG | 626 | In Stock |


|
| 500MG | 1332 | In Stock |


|
| 1G | Get Quote | In Stock |


|
Biological Information
-
Product NameTreosulfan
-
NoteResearch use only, not for human use.
-
Brief DescriptionTreosulfan is a bifunctional alkylating agent. It also has activity in ovarian cancer and other solid tumor types.
-
DescriptionTreosulfan is a bifunctional alkylating agent. It also has activity in ovarian cancer and other solid tumor types. (In Vitro):Treosulfan is an alkylating agent. Treosulfan inhibits several cancer cell lines, such as Panc-1, Miapaca-2 and Capan-2 cells, with IC50s of 3.6 μg/mL, 1.8 μg/mL and 2.1 μg/mL respectively, and shows nearly 100% cytotoxicity on these cell lines at 100 μg/mL. Treosulfan (0.1-100 μg/mL) in combination with LY 188011 exhibits enhanced activity against cancer cells. However, Treosulfan (1, 2.5, 5 μg/ml) combined with 5-FU (0.1, 0.25, 0.5 μg/ml) has antagonistic effect on Panc-1 cells at intermediate and high concentrations, and on Miapaca-2 cells at all doses. Treosulfan (800 μg/mL) dramatically reduces erythrocyte forward scatter, increases the percentage of annexin-V-binding cells, [Ca2+]i, and ROS. Removal of extracellular Ca2+ abrogates the effect of Treosulfan on annexin-V-binding.(In Vivo):Treosulfan (1.5 g/kg/day) induces a rapid myeloablation, depletes the splenic B and T cells in mice. Treosulfan (1.5 g/kg/day) causes olny interleukin-2 production in spleen cells for a short time and without obvious significant effect on synthesis of tumor necrosis factor-α and/or IFN-γ in mice.
-
In VitroTreosulfan is an alkylating agent. Treosulfan inhibits several cancer cell lines, such as Panc-1, Miapaca-2 and Capan-2 cells, with IC50s of 3.6 μg/mL, 1.8 μg/mL and 2.1 μg/mL respectively, and shows nearly 100% cytotoxicity on these cell lines at 100 μg/mL. Treosulfan (0.1-100 μg/mL) in combination with LY 188011 exhibits enhanced activity against cancer cells. However, Treosulfan (1, 2.5, 5 μg/ml) combined with 5-FU (0.1, 0.25, 0.5 μg/ml) has antagonistic effect on Panc-1 cells at intermediate and high concentrations, and on Miapaca-2 cells at all doses. Treosulfan (800 μg/mL) dramatically reduces erythrocyte forward scatter, increases the percentage of annexin-V-binding cells, [Ca2+]i, and ROS. Removal of extracellular Ca2+ abrogates the effect of Treosulfan on annexin-V-binding.
-
In VivoTreosulfan (1.5 g/kg/day) induces a rapid myeloablation, depletes the splenic B and T cells in mice. Treosulfan (1.5 g/kg/day) causes olny interleukin-2 production in spleen cells for a short time and without obvious significant effect on synthesis of tumor necrosis factor-α and/or IFN-γ in mice.
-
SynonymsNSC 39069 | Treosulphan
-
PathwayCell Cycle/DNA Damage
-
TargetDNA/RNA Synthesis
-
RecptorDNA Alkylator
-
Research Area——
-
Indication——
Chemical Information
-
CAS Number299-75-2
-
Formula Weight278.3
-
Molecular FormulaC6H14O8S2
-
Purity>98% (HPLC)
-
SolubilityDMSO:100 mg/mL (359.32 mM);H2O:50 mg/mL (179.66 mM; Need ultrasonic)
-
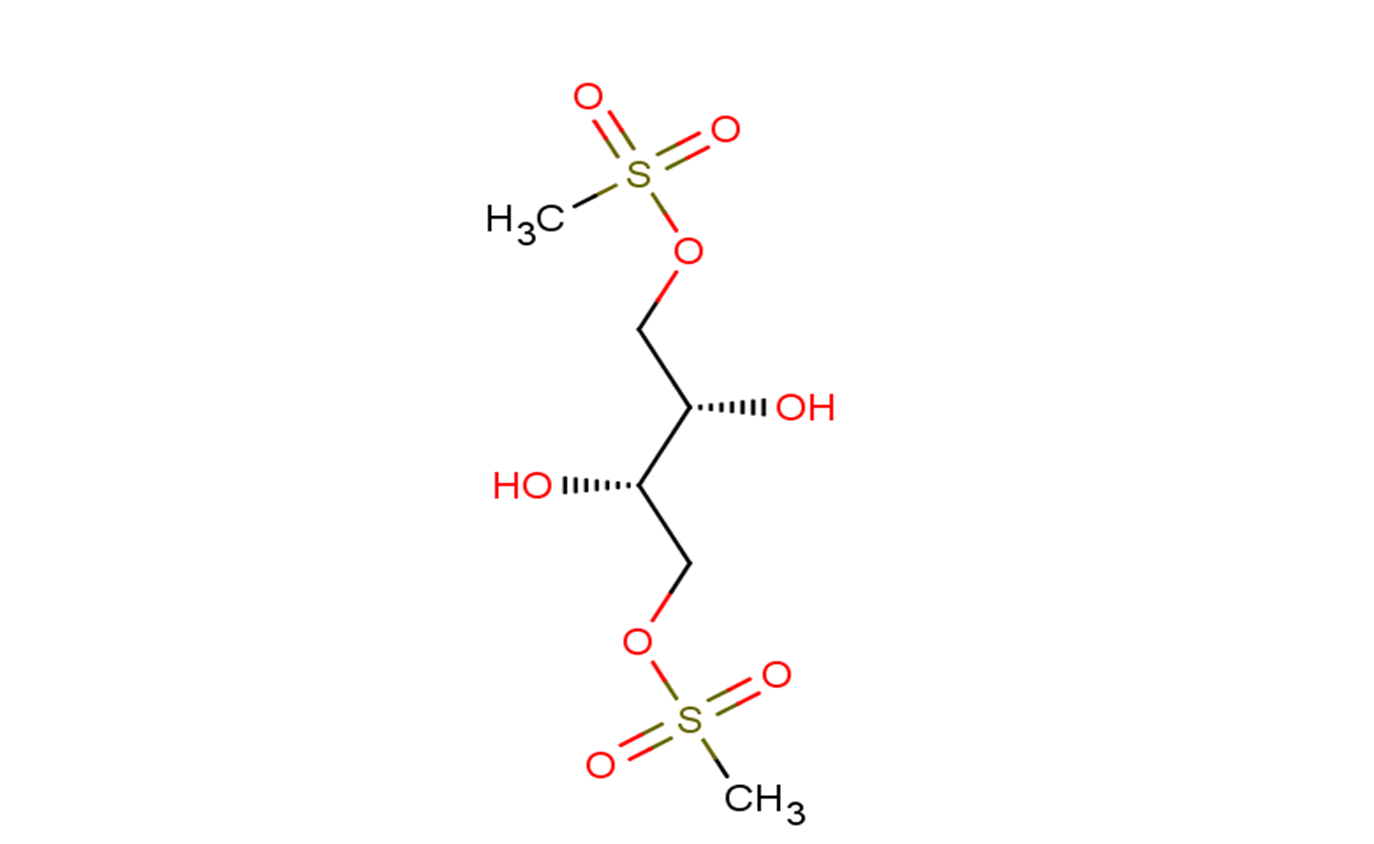
SMILESCS(=O)(=O)OC[C@@H]([C@H](COS(=O)(=O)C)O)O
-
Chemical Name——
Shipping & Storage Information
-
Storage(-20℃)
-
ShippingWith Ice Pack
-
Stability≥ 2 years
Reference
1.Nitsch E, et al. Synergistic cytotoxic activity of treosulfan and LY 188011 in pancreatic cancer cell lines. Anticancer Res. 2014 Apr;34(4):1779-84.
molnova catalog



related products
-
VAL-083
VAL-083 is an alkylating compound that creates N7 methylation on DNA. It also has antitumor activity.
-
2,4-Dichlorophenoxya...
2,4-Dichlorophenoxyacetic acid is a synthetic auxin used as a plant growth regulator and also used as a supplement in plant cell culture media.
-
Sarmustine
Sarmustine (SarCNU) is an alkylating agent with anticancer activity that inhibits the growth of prostate cancer cells via p53-dependent and p53-independent pathways.



 Cart
Cart
 sales@molnova.com
sales@molnova.com


