Tildipirosin
CAS No. 328898-40-4
Tildipirosin( —— )
Catalog No. M20577 CAS No. 328898-40-4
Tildipirosin is a 16-membered macrolide used as an antibiotic in veterinary medicine. Like other macrolides it inhibits protein synthesis in bacteria and blocks the production of biofilms.
Purity : >98% (HPLC)
 COA
COA
 Datasheet
Datasheet
 HNMR
HNMR
 HPLC
HPLC
 MSDS
MSDS
 Handing Instructions
Handing Instructions
| Size | Price / USD | Stock | Quantity |
| 2MG | 88 | In Stock |


|
| 5MG | 178 | In Stock |


|
| 10MG | 295 | In Stock |


|
| 25MG | 502 | In Stock |


|
| 50MG | 709 | In Stock |


|
| 100MG | 1008 | In Stock |


|
| 500MG | 2007 | In Stock |


|
| 1G | Get Quote | In Stock |


|
Biological Information
-
Product NameTildipirosin
-
NoteResearch use only, not for human use.
-
Brief DescriptionTildipirosin is a 16-membered macrolide used as an antibiotic in veterinary medicine. Like other macrolides it inhibits protein synthesis in bacteria and blocks the production of biofilms.
-
DescriptionTildipirosin is a 16-membered macrolide used as an antibiotic in veterinary medicine. Like other macrolides it inhibits protein synthesis in bacteria and blocks the production of biofilms.
-
In VitroTildipirosin exhibits the inhibitory effect on C. coli species, and 23 of 31 (74%) isolates have MICs of 8 or 16 μg/mL while 8 of 31 (26%) have MIC >256 μg/mL. MICs against C. jejuni are 8-64 μg/mL. Tildipirosin against S. enterica and E. coli are 2-8 μg/mL. Tildipirosin inhibits the treponeme isolates form from CODD lesions from 19 sheep, with MIC90 of 0.0469 mg/L. The P. multocida B130 clones show the MIC of 0.25 mg/L for tildipirosin. The 10 P. multocida isolates that carry only erm(42) exhibit MIC of 16-32 mg/L for tildipirosin. The single M. haemolytica that harbours only erm(42) shows MIC of 32 mg/L for tildipirosin.
-
In VivoThe mean percentage of lung consolidation for tildipirosin (4 mg/kg, s.c.)-treated calves is significantly lower than those for tulathromycin-treated and control calves. Metaphylactic administration of tildipirosin to calves 5 days prior to H somni challenge prevents subsequent culture of the pathogen from bronchial secretions and is more effective in minimizing clinical disease and lung lesions than is metaphylactic administration of tulathromycin.
-
Synonyms——
-
PathwayGPCR/G Protein
-
TargetAntibacterial
-
RecptorBacterial
-
Research Area——
-
Indication——
Chemical Information
-
CAS Number328898-40-4
-
Formula Weight734.02
-
Molecular FormulaC41H71N3O8
-
Purity>98% (HPLC)
-
SolubilityDMSO:100 mg/mL (136.24 mM)
-
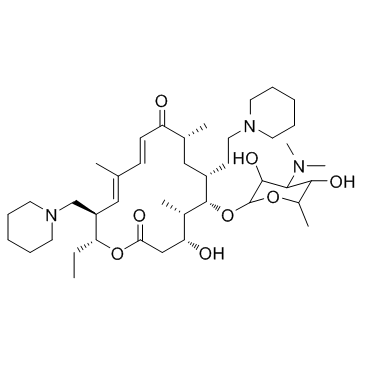
SMILESCC[C@H]1OC(=O)C[C@@H](O)[C@H](C)[C@@H](O[C@@H]2O[C@H](C)[C@@H](O)[C@@H]([C@H]2O)N(C)C)[C@@H](CCN2CCCCC2)C[C@@H](C)C(=O)\C=C\C(\C)=C\[C@@H]1CN1CCCCC1
-
Chemical Name(5S6S7R9R11E13E15R16R)-6-(((2R3R4S5S6R)-4-(dimethylamino)-35-dihydroxy-6-methyltetrahydro-2H-pyran-2-yl)oxy)-16-ethyl-4-hydroxy-5913-trimethyl-7-(2-(piperidin-1-yl)ethyl)-15-(piperidin-1-ylmethyl)oxacyclohexadeca-1113-diene-210-dione
Shipping & Storage Information
-
Storage(-20℃)
-
ShippingWith Ice Pack
-
Stability≥ 2 years
Reference
1.Andersen N M Poehlsgaard J Warrass R et al. Inhibition of Protein Synthesis on the Ribosome by Tildipirosin Compared with Other Veterinary Macrolides[J]. Antimicrobial Agents and Chemotherapy 2012 56(11):6033-6036.
molnova catalog



related products
-
CysHHC10
CysHHC10 is a synthetic antimicrobial peptide (AMP), and exhibits strong anti-microbial properties against both Gram-positive and Gram-negative bacteria.
-
Isovalerylshikonin
Isovalerylshikonin is a natural product from Onosma heterophylla and Arnebia euchroma.
-
Fosfomycin calcium
Fosfomycin calcium is an antibiotics, used in urinary tract infections and intestinal infections caused by susceptible strains.



 Cart
Cart
 sales@molnova.com
sales@molnova.com


