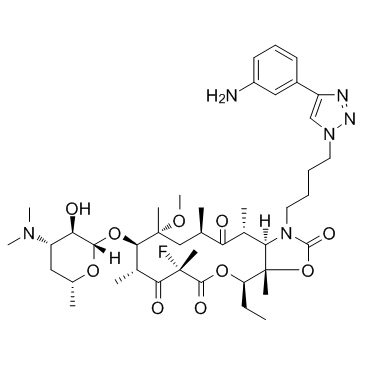
Solithromycin
CAS No. 760981-83-7
Solithromycin( CEM-101 | OP-1068 )
Catalog No. M15888 CAS No. 760981-83-7
A novel fluoroketolide with lower MICs than those of telithromycin and macrolides.
Purity : >98% (HPLC)
 COA
COA
 Datasheet
Datasheet
 HNMR
HNMR
 HPLC
HPLC
 MSDS
MSDS
 Handing Instructions
Handing Instructions
| Size | Price / USD | Stock | Quantity |
| 2MG | 29 | In Stock |


|
| 5MG | 46 | In Stock |


|
| 10MG | 80 | In Stock |


|
| 25MG | 160 | In Stock |


|
| 50MG | 264 | In Stock |


|
| 100MG | Get Quote | In Stock |


|
| 200MG | Get Quote | In Stock |


|
| 500MG | Get Quote | In Stock |


|
| 1G | Get Quote | In Stock |


|
Biological Information
-
Product NameSolithromycin
-
NoteResearch use only, not for human use.
-
Brief DescriptionA novel fluoroketolide with lower MICs than those of telithromycin and macrolides.
-
DescriptionA novel fluoroketolide with lower MICs than those of telithromycin and macrolides; binds to the large 50S subunit of the ribosome and inhibits protein biosynthesis; exhibits MICs of 7.5, 40, and 125 ng/ml for Streptococcus pneumoniae,Staphylococcus aureus, and Haemophilus influenzae, respectively. Bacterial Infection Approved(In Vitro):The IC50s values for Solithromycin on TNFα and CXCL8 release are 41.6 μM and 78.2 μM, respectively. Solithromycin markedly reduces MMP9 activity, with an IC50 of 14.9 μM.Solithromycin (0-333 μM; 72 hours; U937 and PBMC cells) suppresses lipopolysaccharide-induced TNFα release and phorbol 12-myristate 13-acetate (PMA)-induced matrix metalloproteinase 9 (MMP9) activity, and does not affect cell viability in monocytic U937 and PBMC cells.(In Vivo):Solithromycin (100 mg/kg; oral administration; every day; for 8 days; C57BL/6J mice) treatment inhibits inflammatory cells accumulation and pro-MMP9 production in cigarette smoke-exposed mice.
-
In VitroThe IC50s values for Solithromycin on TNFα and CXCL8 release are 41.6 μM and 78.2 μM, respectively. Solithromycin markedly reduces MMP9 activity, with an IC50 of 14.9 μM.Solithromycin (0-333 μM; 72 hours; U937 and PBMC cells) suppresses lipopolysaccharide-induced TNFα release and phorbol 12-myristate 13-acetate (PMA)-induced matrix metalloproteinase 9 (MMP9) activity, and does not affect cell viability in monocytic U937 and PBMC cells.
-
In VivoSolithromycin (100 mg/kg; oral administration; every day; for 8 days; C57BL/6J mice) treatment inhibits inflammatory cells accumulation and pro-MMP9 production in cigarette smoke-exposed mice. Animal Model:C57BL/6J mice (male, 4 weeks)Dosage:100 mg/kg Administration:Oral administration; every day; for 8 days Result:Inhibited cigarette smoke-induced neutrophilia and pro-MMP9 production.
-
SynonymsCEM-101 | OP-1068
-
PathwayGPCR/G Protein
-
TargetAntibacterial
-
RecptorAntibiotic
-
Research AreaInfection
-
IndicationBacterial Infection
Chemical Information
-
CAS Number760981-83-7
-
Formula Weight845.0088
-
Molecular FormulaC43H65FN6O10
-
Purity>98% (HPLC)
-
SolubilityDMSO: ≥ 32 mg/mL
-
SMILESCC[C@@H]1[C@@]2([C@@H]([C@@H](C(=O)[C@@H](C[C@@]([C@@H]([C@H](C(=O)[C@@](C(=O)O1)(C)F)C)O[C@H]3[C@@H]([C@H](C[C@H](O3)C)N(C)C)O)(C)OC)C)C)N(C(=O)O2)CCCCN4C=C(N=N4)C5=CC(=CC=C5)N)C
-
Chemical Name2H-Oxacyclotetradecino[4,3-d]oxazole-2,6,8,14(1H,7H,9H)-tetrone, 1-[4-[4-(3-aminophenyl)-1H-1,2,3-triazol-1-yl]butyl]-4-ethyl-7-fluorooctahydro-11-methoxy-3a,7,9,11,13,15-hexamethyl-10-[[3,4,6-trideoxy-3-(dimethylamino)-β-D-xylo-hexopyranosyl]oxy]-, (3aS,
Shipping & Storage Information
-
Storage(-20℃)
-
ShippingWith Ice Pack
-
Stability≥ 2 years
Reference
1. Waites KB, et al. Antimicrob Agents Chemother. 2009 May;53(5):2139-41.
2. Lemaire S, et al. Antimicrob Agents Chemother. 2009 Sep;53(9):3734-43.
3. Roblin PM, et al. Antimicrob Agents Chemother. 2010 Mar;54(3):1358-9.
molnova catalog



related products
-
Cefmenoxime hydrochl...
Cefmenoxime hydrochloride is a third-generation cephalosporin antibiotic.
-
Nacubactam
Nacubactam is a potent inhibitor of non-β-lactam-β-lactamase with activity against class A and class C β-lactamases.
-
Sulfisoxazole acetyl
Sulfisoxazole acetyl is an agent with antibacterial activity. Sulfisoxazole acetyl is a Sulfisoxazole derivative with antibacterial activity. Sulfisoxazole acetyl is an inhibitor of dihydropteroate synthase.



 Cart
Cart
 sales@molnova.com
sales@molnova.com


