Ro-3306
CAS No. 872573-93-8
Ro-3306( Ro3306 )
Catalog No. M16345 CAS No. 872573-93-8
Ro-3306 is a potent, selective, ATP-competitive CDK1 inhibitor with Ki of 35 nM against CDK1/cyclin B1, 10-fold selectivity relative to CDK2/cyclin E and >50-fold relative to CDK4/cyclin D.
Purity : >98% (HPLC)
 COA
COA
 Datasheet
Datasheet
 HNMR
HNMR
 HPLC
HPLC
 MSDS
MSDS
 Handing Instructions
Handing Instructions
| Size | Price / USD | Stock | Quantity |
| 5MG | 61 | In Stock |


|
| 10MG | 84 | In Stock |


|
| 25MG | 142 | In Stock |


|
| 50MG | 205 | In Stock |


|
| 100MG | 335 | In Stock |


|
| 500MG | 782 | In Stock |


|
| 1G | Get Quote | In Stock |


|
Biological Information
-
Product NameRo-3306
-
NoteResearch use only, not for human use.
-
Brief DescriptionRo-3306 is a potent, selective, ATP-competitive CDK1 inhibitor with Ki of 35 nM against CDK1/cyclin B1, 10-fold selectivity relative to CDK2/cyclin E and >50-fold relative to CDK4/cyclin D.
-
DescriptionRo-3306 is a potent, selective, ATP-competitive CDK1 inhibitor with Ki of 35 nM against CDK1/cyclin B1, 10-fold selectivity relative to CDK2/cyclin E and >50-fold relative to CDK4/cyclin D; also inhibits CDK1/cyclin A complexes with Ki of 110 nM, shows >15-fold selectivity against a diverse panel of 8 human kinases; reversibly arrests human cells at the G(2)/M border of the cell cycle and allows for effective cell synchronization in early mitosis; enhances p53-mediated Bax activation and mitochondrial apoptosis in AML.
-
In VitroRO-3306 is an ATP-competitive inhibitor, and inhibits CDK1/cyclin A complexes with Ki of 110 nM. RO-3306 blocks the cell cycle in the G2/M phase of human cancer cells. RO-3306 (4 μM) induces apoptosis in cancer cells. RO-3306 (5 μM) induces G2/M-phase cell cycle arrest and apoptosis of AML cells in a time-dependent manner. RO-3306 treatment significantly increases the percentage of Annexin V-positive cells in G1-phase cells without affecting the cell cycle distribution. RO-3306 enhances p53-mediated apoptosis. RO-3306 cooperates with Nutlin-3 in activating Bax and inducing mitochondrial apoptosis. RO-3306 (5 μM) downregulates antiapoptotic p21, Bcl-2 and survivin protein expression in AML. RO-3306 inhibits p53-induced p21 synthesis. RO-3306 does not inhibit RNA polymerase II CTD phosphorylation. RO-3306 (10 μM) effectively arrests oocyte maturation. RO-3306 reduces the blastocyst formation in oocytes.
-
In Vivo——
-
SynonymsRo3306
-
PathwayAngiogenesis
-
TargetCDK
-
RecptorCDK1|ERK|PKA|PKCδ|SGK
-
Research AreaCancer
-
Indication——
Chemical Information
-
CAS Number872573-93-8
-
Formula Weight351.4453
-
Molecular FormulaC18H13N3OS2
-
Purity>98% (HPLC)
-
SolubilityDMSO: ≥ 47 mg/mL
-
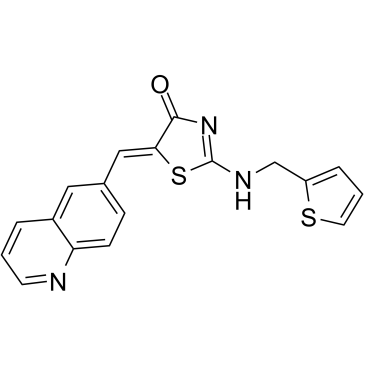
SMILESO=C1N=C(NCC2=CC=CS2)S/C1=C/C3=CC=C4N=CC=CC4=C3
-
Chemical Name4(5H)-Thiazolone, 5-(6-quinolinylmethylene)-2-[(2-thienylmethyl)amino]-, (5Z)-
Shipping & Storage Information
-
Storage(-20℃)
-
ShippingWith Ice Pack
-
Stability≥ 2 years
Reference
1. Vassilev LT, et al. Proc Natl Acad Sci U S A. 2006 Jul 11;103(28):10660-5.
2. Kojima K, et al. Cancer Sci. 2009 Jun;100(6):1128-36.
3. Krasinska L, et al. Cell Cycle. 2008 Jun 15;7(12):1702-8.
4. Vassilev LT. Cell Cycle. 2006 Nov;5(22):2555-6.
molnova catalog



related products
-
LY2835219 mesylate
LY2835219 is a potent and selective inhibitor of CDK4 and CDK6 with IC50 of 2 nM and 10 nM in cell-free assays, respectively. Phase 3.
-
CDK inhibitor E9
CDK inhibitor E9 is a novel CDK inhibitor that can overcomes ABC-mediated resistance of THZ1, functions as a potent, non-covalent inhibitor of CDK9 and a covalent inhibitor of CDK12.
-
CAN-508
CAN-508 is a potent, selective CDK9 inhibitor with IC50 of 0.35 uM (CDK9/cyclin T1), displays >35 fold selectivity over CDK1/2/4/7 (IC50=13.5-70 uM).



 Cart
Cart
 sales@molnova.com
sales@molnova.com


