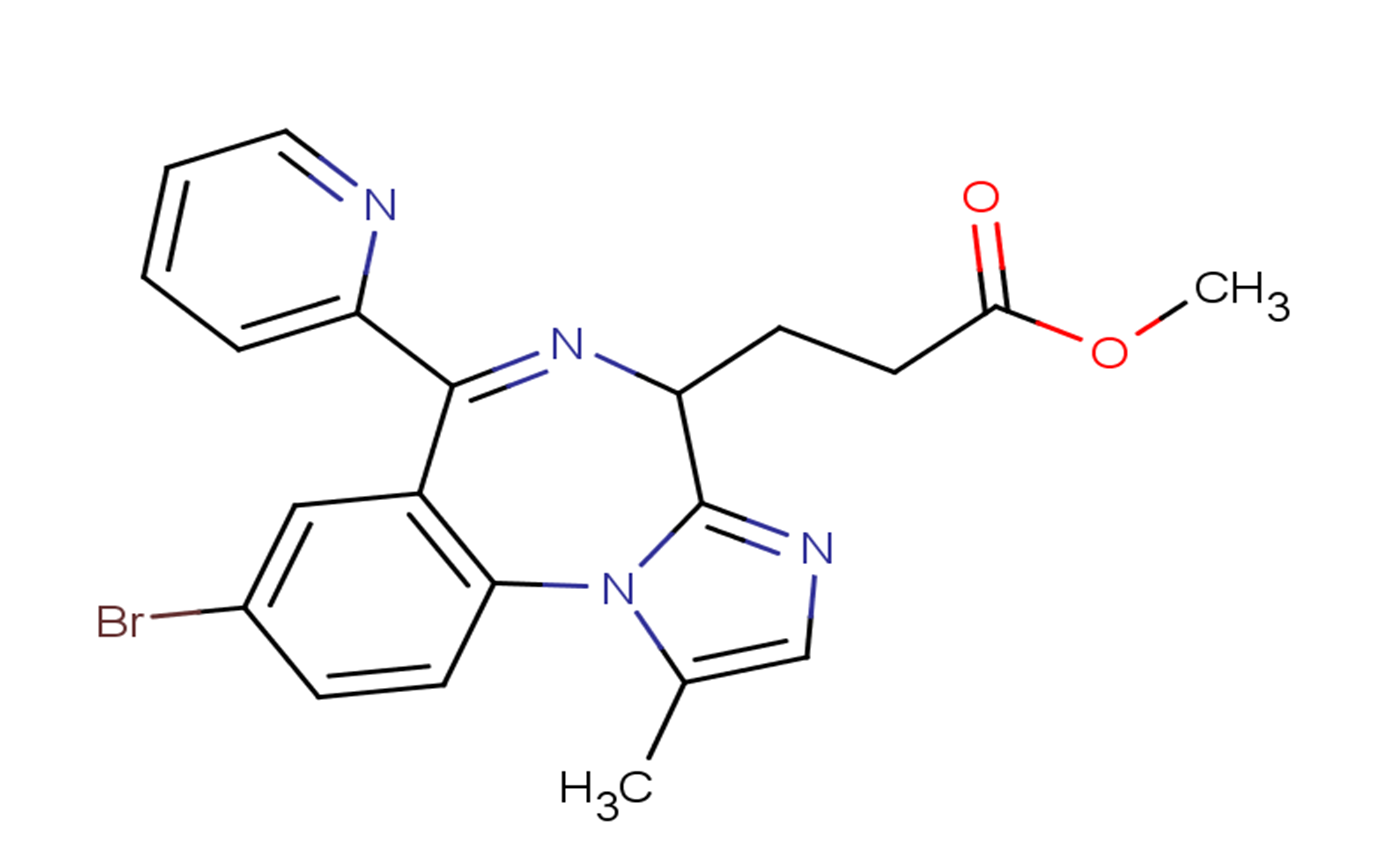
Remimazolam
CAS No. 308242-62-8
Remimazolam( CNS-7056,CNS 7056,CNS7056 )
Catalog No. M22876 CAS No. 308242-62-8
Remimazolam is a benzodiazepine derivative drug as an alternative to the short-acting imidazobenzodiazepine midazolam, for use in induction of anaesthesia and conscious sedation for minor invasive procedures.?Remimazolam was found to be both faster acting and shorter lasting than midazolam, and human clinical trials showed a faster recovery time and predictable, consistent pharmacokinetics, suggesting some advantages over existing drugs for these applications.?
Purity : >98% (HPLC)
 COA
COA
 Datasheet
Datasheet
 HNMR
HNMR
 HPLC
HPLC
 MSDS
MSDS
 Handing Instructions
Handing Instructions
| Size | Price / USD | Stock | Quantity |
| 5MG | 357 | In Stock |


|
| 10MG | 596 | In Stock |


|
| 25MG | 882 | In Stock |


|
| 50MG | Get Quote | In Stock |


|
| 100MG | Get Quote | In Stock |


|
| 200MG | Get Quote | In Stock |


|
| 500MG | Get Quote | In Stock |


|
| 1G | Get Quote | In Stock |


|
Biological Information
-
Product NameRemimazolam
-
NoteResearch use only, not for human use.
-
Brief DescriptionRemimazolam is a benzodiazepine derivative drug as an alternative to the short-acting imidazobenzodiazepine midazolam, for use in induction of anaesthesia and conscious sedation for minor invasive procedures.?Remimazolam was found to be both faster acting and shorter lasting than midazolam, and human clinical trials showed a faster recovery time and predictable, consistent pharmacokinetics, suggesting some advantages over existing drugs for these applications.?
-
DescriptionRemimazolam is a benzodiazepine derivative drug as an alternative to the short-acting imidazobenzodiazepine midazolam, for use in induction of anaesthesia and conscious sedation for minor invasive procedures.?Remimazolam was found to be both faster acting and shorter lasting than midazolam, and human clinical trials showed a faster recovery time and predictable, consistent pharmacokinetics, suggesting some advantages over existing drugs for these applications.?Mice showed significantly increased time to movement outside a set perimeter after 5-minute exposure to increasing concentrations (10-25 mg/mL solutions) of inhaled remimazolam aerosols.?Differences in mean (95% confidence interval) time to movement from pretest baseline group (0.05 [0.01-0.09] minutes) were 11 (4-18), 15 (5-26), 30 (19-41), and 109 (103-115) minutes after exposure to remimazolam aerosol of 10, 15, 20, and 25 mg/mL, respectively (P = .007 - P
-
In Vitro——
-
In Vivo——
-
SynonymsCNS-7056,CNS 7056,CNS7056
-
PathwayOthers
-
TargetOther Targets
-
RecptorOthers
-
Research Areanervous system
-
IndicationAnesthesia
Chemical Information
-
CAS Number308242-62-8
-
Formula Weight439.31
-
Molecular FormulaC21H19BrN4O2
-
Purity>98% (HPLC)
-
SolubilityDMSO:Soluble; Water:Insoluble
-
SMILESCC1=CN=C2N1C3=C(C=C(C=C3)Br)C(=NC2CCC(=O)OC)C4=CC=CC=N4
-
Chemical Name——
Shipping & Storage Information
-
Storage(-20℃)
-
ShippingWith Ice Pack
-
Stability≥ 2 years
Reference
1. Rower, Joseph, Sakata, et al. Inhaled Remimazolam Potentiates Inhaled Remifentanil in Rodents[J]. Anesthesia and Analgesia: Journal of the International Anesthesia Research Society, 2017.
molnova catalog



related products
-
Thalidomide-NH-C2-PE...
Thalidomide-NH-C2-PEG3-OH is an E3 ligase ligand-linker conjugate.
-
3-O-Feruloylquinic a...
3-O-Feruloylquinic acid is a protease inhibitor, it exerts moderate inhibitory effect against AIV (H5N1) in vitro.
-
Mulberrofuran Q
Mulberrofuran Q is an active compound that inhibits the formation of 12-hydroxy-5,8,10-heptadecatrienoic acid (HHT) and thromboxane B2 (cyclooxygenase product) , which protects neuronal cells from hypoxia-glycorrhoea-deficiency (OGD)-induced oxidative stress.



 Cart
Cart
 sales@molnova.com
sales@molnova.com


