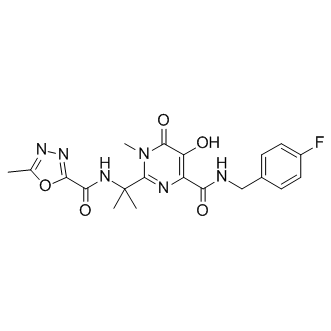
Raltegravir
CAS No. 518048-05-0
Raltegravir( MK-0518 | MK 0518 | MK0518 )
Catalog No. M14807 CAS No. 518048-05-0
A potent, selective, orally bioavailable HIV-integrase inhibitor with IC50 of 15 nM.
Purity : >98% (HPLC)
 COA
COA
 Datasheet
Datasheet
 HNMR
HNMR
 HPLC
HPLC
 MSDS
MSDS
 Handing Instructions
Handing Instructions
| Size | Price / USD | Stock | Quantity |
| 2MG | 34 | In Stock |


|
| 5MG | 52 | In Stock |


|
| 10MG | 95 | In Stock |


|
| 25MG | 173 | In Stock |


|
| 50MG | 286 | In Stock |


|
| 100MG | 426 | In Stock |


|
| 200MG | Get Quote | In Stock |


|
| 500MG | Get Quote | In Stock |


|
| 1G | Get Quote | In Stock |


|
Biological Information
-
Product NameRaltegravir
-
NoteResearch use only, not for human use.
-
Brief DescriptionA potent, selective, orally bioavailable HIV-integrase inhibitor with IC50 of 15 nM.
-
DescriptionA potent, selective, orally bioavailable HIV-integrase inhibitor with IC50 of 15 nM; essentially inactive (IC50s>50 uM) against HCV Pol, HIV RT, HIV RNase-H, and human α/β/γ Pol; shows inhibitory activity against the wild type virus and a selection of mutants; the first HIV-integrase inhibitor for the treatment of HIV-1 infection.HIV Infection Approved(In Vitro):PFV IN carrying the S217H substitution is 10-fold less susceptible to Raltegravir with IC50 of 900 nM. PFV IN displays 10% of WT activity and is inhibited by Raltegravir with an IC50 of 200 nM, indicating a appr twofold decrease in susceptibility to the IN strand transfer inhibitor (INSTI) compared with WT IN. S217Q PFV IN is as sensitive to Raltegravir as the WT enzyme. Raltegravir is metabolized by glucuronidation, not hepatically. Raltegravir has potent in vitro activity against HIV-1, with a 95% inhibitory concentration of 31±20 nM, in human T lymphoid cell cultures. Raltegravir is also active against HIV-2 when Raltegravir is tested in CEMx174 cells, with an IC95of 6 nM. Raltegravir metabolism occurs primarily through glucuronidation. Drugs that are strong inducers of the glucuronidation enzyme, UGT1A1, significantly reduce Raltegravir concentrations and should not be used. Raltegravir exhibits weak inhibitory effects on hepatic cytochrome P450 activity. Raltegravir does not induce CYP3A4 RNA expression or CYP3A4-dependent testosterone 6-β-hydroxylase activity. Raltegravir cellular permeativity is reduced in the presence of magnesium and calcium. Raltegravir and related HIV-1 integrase (IN) strand transfer inhibitors (INSTIs efficiently block viral replication. In acutely infected human lymphoid CD4+ T-cell lines MT-4 and CEMx174, SIVmac251 replication is efficiently inhibited by Raltegravir, which shows an EC90 in the low nanomolar range.(In Vivo):Raltegravir induces viro-immunological improvement of nonhuman primates with progressing SIVmac251 infection. One non-human primate shows an undetectable viral load following Raltegravir monotherapy.
-
In VitroPFV IN carrying the S217H substitution is 10-fold less susceptible to Raltegravir with IC50 of 900 nM. PFV IN displays 10% of WT activity and is inhibited by Raltegravir with an IC50 of 200 nM, indicating a appr twofold decrease in susceptibility to the IN strand transfer inhibitor (INSTI) compared with WT IN. S217Q PFV IN is as sensitive to Raltegravir as the WT enzyme. Raltegravir is metabolized by glucuronidation, not hepatically. Raltegravir has potent in vitro activity against HIV-1, with a 95% inhibitory concentration of 31±20 nM, in human T lymphoid cell cultures. Raltegravir is also active against HIV-2 when Raltegravir is tested in CEMx174 cells, with an IC95of 6 nM. Raltegravir metabolism occurs primarily through glucuronidation. Drugs that are strong inducers of the glucuronidation enzyme, UGT1A1, significantly reduce Raltegravir concentrations and should not be used. Raltegravir exhibits weak inhibitory effects on hepatic cytochrome P450 activity. Raltegravir does not induce CYP3A4 RNA expression or CYP3A4-dependent testosterone 6-β-hydroxylase activity. Raltegravir cellular permeativity is reduced in the presence of magnesium and calcium. Raltegravir and related HIV-1 integrase (IN) strand transfer inhibitors (INSTIs efficiently block viral replication. In acutely infected human lymphoid CD4+ T-cell lines MT-4 and CEMx174, SIVmac251 replication is efficiently inhibited by Raltegravir, which shows an EC90 in the low nanomolar range.
-
In VivoRaltegravir induces viro-immunological improvement of nonhuman primates with progressing SIVmac251 infection. One non-human primate shows an undetectable viral load following Raltegravir monotherapy.
-
SynonymsMK-0518 | MK 0518 | MK0518
-
PathwayMicrobiology/Virology
-
TargetHIV
-
RecptorIntegrase(S217QPFV)|Integrase(WTPFV)
-
Research AreaInfection
-
IndicationHIV Infection
Chemical Information
-
CAS Number518048-05-0
-
Formula Weight444.4164
-
Molecular FormulaC20H21FN6O5
-
Purity>98% (HPLC)
-
Solubility10 mM in DMSO
-
SMILESCC1=NN=C(O1)C(=O)NC(C)(C)C2=NC(=C(C(=O)N2C)O)C(=O)NCC3=CC=C(C=C3)F
-
Chemical Name4-Pyrimidinecarboxamide, N-[(4-fluorophenyl)methyl]-1,6-dihydro-5-hydroxy-1-methyl-2-[1-methyl-1-[[(5-methyl-1,3,4-oxadiazol-2-yl)carbonyl]amino]ethyl]-6-oxo-
Shipping & Storage Information
-
Storage(-20℃)
-
ShippingWith Ice Pack
-
Stability≥ 2 years
Reference
1. Summa V, et al. J Med Chem. 2008 Sep 25;51(18):5843-55.
2. Malet I, et al. Antimicrob Agents Chemother. 2008 Apr;52(4):1351-8.
3. Cooper DA, et al. N Engl J Med. 2008 Jul 24;359(4):355-65.
molnova catalog



related products
-
I-XW-053
I-XW-053 is an inhibitor of capsid targeted HIV-1 replication using the hybrid structure based method to block the interface between CA N-terminal domains (NTD-NTD interface) with micromolar affinity.
-
Fosdevirine
Fosdevirine (GSK2248761) is a selective and potent non-nucleoside reverse transcriptase (NNRTI) inhibitor with anti-HIV activity for the study of neurological related disorders such as late-onset epilepsy.
-
Bictegravir Sodium
Bictegravir Sodium (GS-9883 Sodium) is a potent inhibitor of HIV-1 integrase, with an IC50 of 7.5 nM. Bictegravir Sodium exhibits potent and selective anti-HIV activity and low cytotoxicity



 Cart
Cart
 sales@molnova.com
sales@molnova.com


