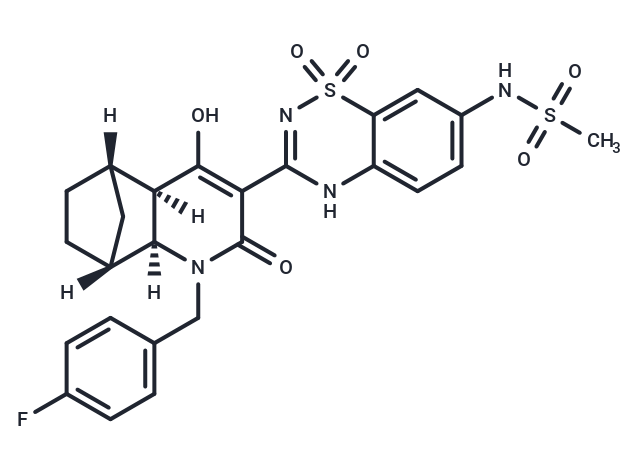
Setrobuvir
CAS No. 1071517-39-9
Setrobuvir( —— )
Catalog No. M34421 CAS No. 1071517-39-9
Setrobuvir (ANA-598) is an orally active non-nucleoside HCV NS5B polymerase inhibitor with inhibitory effects on de novo RNA synthesis and primer extension with IC50s between 4 and 5 nM.
Purity : >98% (HPLC)
 COA
COA
 Datasheet
Datasheet
 HNMR
HNMR
 HPLC
HPLC
 MSDS
MSDS
 Handing Instructions
Handing Instructions
| Size | Price / USD | Stock | Quantity |
| 1 mL x 10 mM in DMSO | 737 | In Stock |


|
| 5MG | 570 | In Stock |


|
| 10MG | 812 | In Stock |


|
| 25MG | 1216 | In Stock |


|
| 50MG | 1628 | In Stock |


|
| 100MG | 2195 | In Stock |


|
| 200MG | Get Quote | In Stock |


|
| 500MG | 4399 | In Stock |


|
| 1G | Get Quote | In Stock |


|
Biological Information
-
Product NameSetrobuvir
-
NoteResearch use only, not for human use.
-
Brief DescriptionSetrobuvir (ANA-598) is an orally active non-nucleoside HCV NS5B polymerase inhibitor with inhibitory effects on de novo RNA synthesis and primer extension with IC50s between 4 and 5 nM.
-
DescriptionSetrobuvir (ANA598) is an orally active non-nucleosidic HCV NS5B polymerase inhibitor. ANA-598 inhibits both de novo RNA synthesis and primer extension, with IC50s between 4 and 5 nM. Setrobuvir also shows excellent binding affinity to SARS-CoV-2 RdRp and induces RdRp inhibition.
-
In VitroSetrobuvir (ANA598) is a non-nucleoside inhibitor that binds to the palm pocket of the HCV polymerase and has an EC50 against HCV genotype 1b/Con1-containing subgenomic replicons in the nanomolar range. Setrobuvir appears to inhibit both de novoinitiated RNA synthesis and primer extension, and its activity is unchanged by the presence of mutations that modify the activity of thumb-binding non-nucleoside inhibitors.
-
In Vivo——
-
Synonyms——
-
PathwayOthers
-
TargetOther Targets
-
RecptorSARS-CoV | DNA/RNA Synthesis | HCV Protease
-
Research Area——
-
Indication——
Chemical Information
-
CAS Number1071517-39-9
-
Formula Weight560.62
-
Molecular FormulaC25H25FN4O6S2
-
Purity>98% (HPLC)
-
Solubility——
-
SMILESC(N1[C@@]2([C@@]([C@@]3(C[C@]2(CC3)[H])[H])(C(O)=C(C1=O)C=4NC=5C(S(=O)(=O)N4)=CC(NS(C)(=O)=O)=CC5)[H])[H])C6=CC=C(F)C=C6
-
Chemical Name——
Shipping & Storage Information
-
Storage(-20℃)
-
ShippingWith Ice Pack
-
Stability≥ 2 years
Reference
1. Yi G, et al. Biochemical study of the comparative inhibition of hepatitis C virus RNA polymerase by VX-222 and filibuvir. Antimicrob Agents Chemother. 2012;56(2):830‐837.?
molnova catalog



related products
-
W-13 hydrochloride
calmodulin antagonist.
-
β-Amyloid/A4 Protein...
β-Amyloid/A4 Protein Precursor 770 (394 - 410)
-
5-Hydroxy-4a,8-dimet...
The herbs of Inula hupehensis.



 Cart
Cart
 sales@molnova.com
sales@molnova.com


