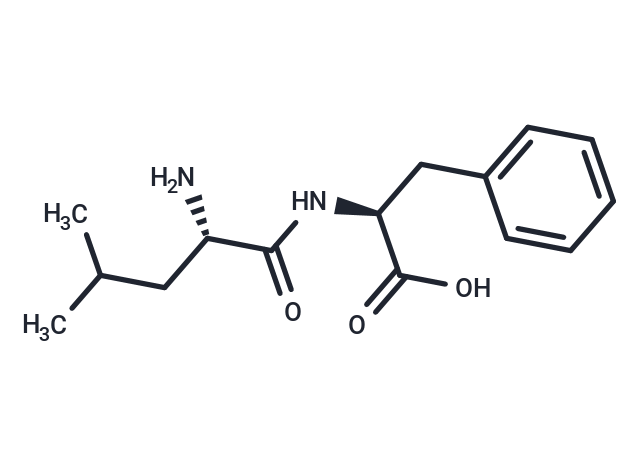
Leucyl-phenylalanine
CAS No. 3063-05-6
Leucyl-phenylalanine( —— )
Catalog No. M33609 CAS No. 3063-05-6
Leucyl-phenylalanine (H-LEU-PHE-OH) is a dipeptide compound that can be used for protein synthesis.
Purity : >98% (HPLC)
 COA
COA
 Datasheet
Datasheet
 HNMR
HNMR
 HPLC
HPLC
 MSDS
MSDS
 Handing Instructions
Handing Instructions
| Size | Price / USD | Stock | Quantity |
| 10MG | 28 | In Stock |


|
| 100MG | Get Quote | In Stock |


|
| 200MG | Get Quote | In Stock |


|
| 500MG | Get Quote | In Stock |


|
| 1G | Get Quote | In Stock |


|
Biological Information
-
Product NameLeucyl-phenylalanine
-
NoteResearch use only, not for human use.
-
Brief DescriptionLeucyl-phenylalanine (H-LEU-PHE-OH) is a dipeptide compound that can be used for protein synthesis.
-
DescriptionLeucyl-phenylalanine belongs to the class of organic compounds known as dipeptides.
-
In Vitro——
-
In Vivo——
-
Synonyms——
-
PathwayProteasome/Ubiquitin
-
TargetEndogenous Metabolite
-
RecptorEndogenous Metabolite
-
Research Area——
-
Indication——
Chemical Information
-
CAS Number3063-05-6
-
Formula Weight278.35
-
Molecular FormulaC15H22N2O3
-
Purity>98% (HPLC)
-
SolubilityIn Vitro:?H2O : 4 mg/mL (14.37 mM; Ultrasonic)Ethanol : 1.11 mg/mL (3.99 mM; Ultrasonic)DMSO : < 1 mg/mL (insoluble or slightly soluble)
-
SMILESC([C@H](NC([C@H](CC(C)C)N)=O)C(O)=O)C1=CC=CC=C1
-
Chemical Name——
Shipping & Storage Information
-
Storage(-20℃)
-
ShippingWith Ice Pack
-
Stability≥ 2 years
Reference
molnova catalog



related products
-
Tetradecanoylcarniti...
Tetradecanoylcarnitine (L-Myristoylcarnitine) is an acylcarnitine involved in the beta-oxidation of long-chain fatty acids, and is a potential marker for very-long-chain coenzyme A dehydrogenase deficiency.
-
4-Hydroxybenzylamine
4-HOBA is a less reactive isomer of 2-HOBA as well as related compounds in a mouse model of hypertension.
-
Glyceryl trimyristat...
Within the cell glyceryl trimyristate is primarily located in the membrane (predicted from logP) and adiposome. glyceryl trimyristate exists in all eukaryotes ranging from yeast to humans. In humans glyceryl trimyristate is involved in the metabolic disorder called de novo triacylglycerol biosynthesis glyceryl trimyristate pathway.



 Cart
Cart
 sales@molnova.com
sales@molnova.com


