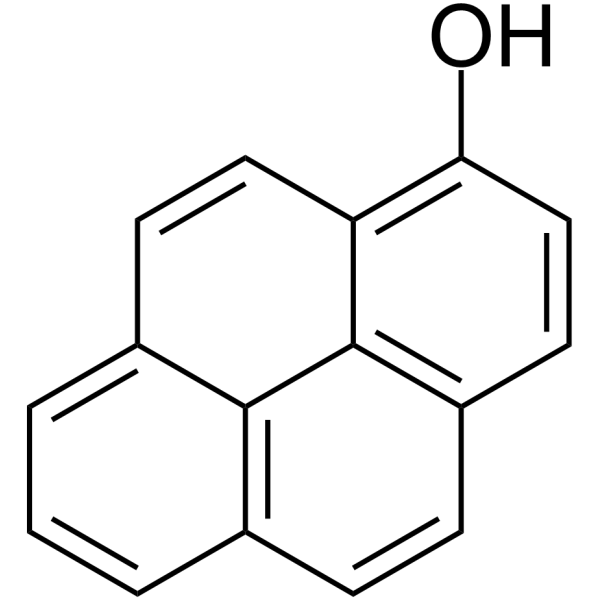
1-Hydroxypyrene
CAS No. 5315-79-7
1-Hydroxypyrene( Pyren-1-ol | 1-Pyrenol )
Catalog No. M27047 CAS No. 5315-79-7
1-Hydroxypyrene, a biomarker of exposure to polycyclic aromatic hydrocarbons, is analyzed in urine samples.
Purity : >98% (HPLC)
 COA
COA
 Datasheet
Datasheet
 HNMR
HNMR
 HPLC
HPLC
 MSDS
MSDS
 Handing Instructions
Handing Instructions
| Size | Price / USD | Stock | Quantity |
| 1 mL x 10 mM in DMSO | 28 | In Stock |


|
| 100MG | Get Quote | In Stock |


|
| 200MG | Get Quote | In Stock |


|
| 500MG | 37 | In Stock |


|
| 1G | 45 | In Stock |


|
Biological Information
-
Product Name1-Hydroxypyrene
-
NoteResearch use only, not for human use.
-
Brief Description1-Hydroxypyrene, a biomarker of exposure to polycyclic aromatic hydrocarbons, is analyzed in urine samples.
-
Description1-Hydroxypyrene, a biomarker of exposure to polycyclic aromatic hydrocarbons, is analyzed in urine samples.
-
In Vitro——
-
In Vivo——
-
SynonymsPyren-1-ol | 1-Pyrenol
-
PathwayProteasome/Ubiquitin
-
TargetEndogenous Metabolite
-
RecptorERβ
-
Research Area——
-
Indication——
Chemical Information
-
CAS Number5315-79-7
-
Formula Weight218.255
-
Molecular FormulaC16H10O
-
Purity>98% (HPLC)
-
SolubilityIn Vitro:?DMSO : 50 mg/mL (229.08 mM)
-
SMILESOc1ccc2ccc3cccc4ccc1c2c34
-
Chemical Name——
Shipping & Storage Information
-
Storage(-20℃)
-
ShippingWith Ice Pack
-
Stability≥ 2 years
Reference
1.Gregory Lee Durst, et al. Substituted benzopyrans as selective estrogen receptor-beta agonists. EP1626974B1
molnova catalog



related products
-
Levomefolic Acid
Levomefolinic acid is a natural active form of folic acid used at the cellular level for DNA reproduction the cysteine cycle and the regulation of homocysteine among other functions.?Levomefolinic acid is used as a treatment and to prevent Folate Deficiency.
-
2-Methylbutyraldehyd...
2-Methylbutanal belongs to the class of organic compounds known as short-chain aldehydes.
-
3-Hydroxyglutaric ac...
3-Hydroxyglutaric acid is one of several metabolites produced when insufficient levels of GCDH are available. It is used as a biomarker of GCDH deficiency.



 Cart
Cart
 sales@molnova.com
sales@molnova.com


