SID 26681509
CAS No. 958772-66-2
SID 26681509( —— )
Catalog No. M26442 CAS No. 958772-66-2
SID 26681509 is a selective, reversible and competitive human cathepsin L inhibitor (IC50 of 56 nM).
Purity : >98% (HPLC)
 COA
COA
 Datasheet
Datasheet
 HNMR
HNMR
 HPLC
HPLC
 MSDS
MSDS
 Handing Instructions
Handing Instructions
| Size | Price / USD | Stock | Quantity |
| 1 mL x 10 mM in DMSO | 166 | In Stock |


|
| 5MG | 140 | In Stock |


|
| 10MG | 217 | In Stock |


|
| 25MG | 436 | In Stock |


|
| 50MG | 639 | In Stock |


|
| 100MG | 910 | In Stock |


|
| 200MG | Get Quote | In Stock |


|
| 500MG | Get Quote | In Stock |


|
| 1G | Get Quote | In Stock |


|
Biological Information
-
Product NameSID 26681509
-
NoteResearch use only, not for human use.
-
Brief DescriptionSID 26681509 is a selective, reversible and competitive human cathepsin L inhibitor (IC50 of 56 nM).
-
DescriptionSID 26681509 is a selective, reversible and competitive human cathepsin L inhibitor (IC50 of 56 nM).(In Vitro):After a 4 hr preincubation with cathepsin L, SID 26681509 becomes more potent(IC50 : 1.0 nM). SID 26681509 is determined to be a slow-binding and slowly reversible competitive inhibitor. Through a transient kinetic analysis for single-step reversibility, inhibition rate constants are kon = 24,000 M-1s-1 and koff = 2.2 × 10-5 s-1 (Ki = 0.89 nM). Molecular docking studies are undertaken using the experimentally-derived X-ray crystal structure of papain/CLIK-148. SID 26681509 inhibits papain and cathepsins B, K, S, and V with IC50 values determined after one hour ranging from 618 nM to 8.442 μM.(In Vivo):survival in murine models of sepsis significantly improved SID 26681509 by and reduces liver damage following warm liver ischemia/reperfusion (I/R) models.
-
In VitroAfter a 4 hr preincubation with cathepsin L, SID 26681509 becomes more potent, demonstrating an IC50 of 1.0 nM. SID 26681509 is determined to be a slow-binding and slowly reversible competitive inhibitor. Through a transient kinetic analysis for single-step reversibility, inhibition rate constants are kon = 24,000 M-1s-1 and koff = 2.2 × 10-5 s-1 (Ki = 0.89 nM). Molecular docking studies are undertaken using the experimentally-derived X-ray crystal structure of papain/CLIK-148. SID 26681509 inhibits papain and cathepsins B, K, S, and V with IC50 values determined after one hour ranging from 618 nM to 8.442 μM. SID 26681509 shows no inhibitory activity against the serine protease cathepsin G. SID 26681509 inhibits cathepsin V activity with an IC50 value of 0.5 μM. SID 26681509 (1-30 μM) blocks high-mobility group box 1 (HMGB1)-induced TNF-α production dose dependently without altering cell viability.
-
In VivoSID 26681509 treatment significantly improves survival in murine models of sepsis and reduces liver damage following warm liver ischemia/reperfusion (I/R) models.
-
Synonyms——
-
PathwayProteasome/Ubiquitin
-
TargetCysteine Protease
-
RecptorHormone-sensitive lipase (HSL)
-
Research Area——
-
Indication——
Chemical Information
-
CAS Number958772-66-2
-
Formula Weight539.65
-
Molecular FormulaC27H33N5O5S
-
Purity>98% (HPLC)
-
SolubilityIn Vitro:?DMSO : 50 mg/mL (92.65 mM)
-
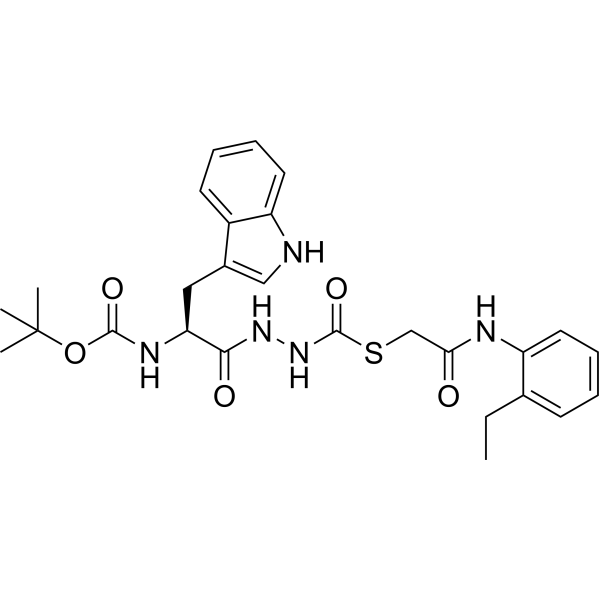
SMILESCCc1ccccc1NC(=O)CSC(=O)NNC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)OC(C)(C)C
-
Chemical Name——
Shipping & Storage Information
-
Storage(-20℃)
-
ShippingWith Ice Pack
-
Stability≥ 2 years
Reference
1.Ebdrup, Soren, et al. PHARMACEUTICAL USE OF BORONIC ACIDS AND ESTERS THEREOF. US7037905.
molnova catalog



related products
-
2-cyano-Pyrimidine
2-cyano-Pyrimidine is inhibitor of cathepsin K(IC50 : 170 nM).
-
Isovalerylcarnitine
Isovalerylcarnitine (3-methylbutyrylcarnitine) is a small molecule compound produced by the catabolism of L-leucine and the accumulation of isovaleric acid.
-
L 006235
L 006235 is a potent and reversible inhibitor of cathepsin K with IC50 of 0.25 nM.



 Cart
Cart
 sales@molnova.com
sales@molnova.com


