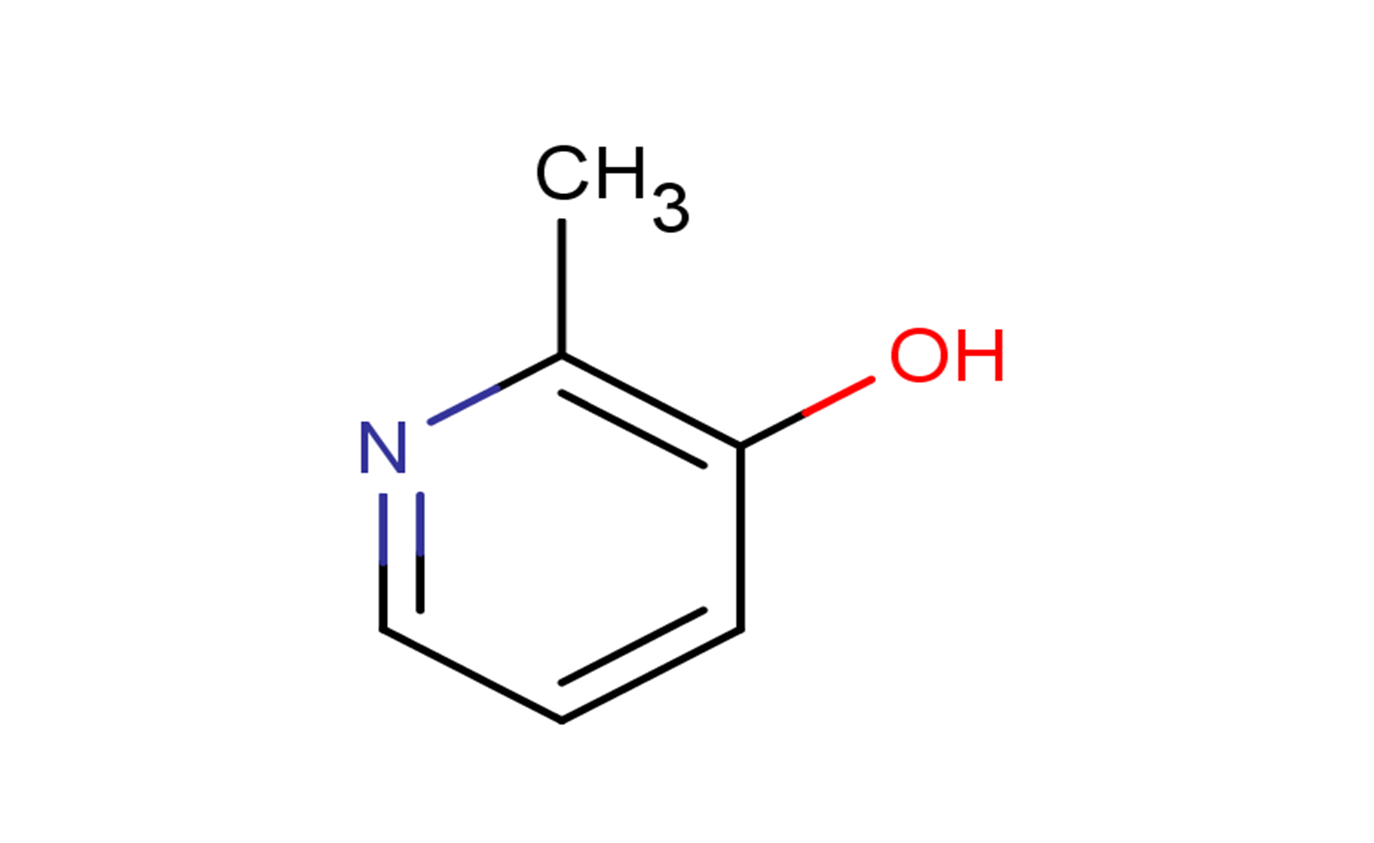
3-Hydroxy-2-methylpyridine
CAS No. 1121-25-1
3-Hydroxy-2-methylpyridine( —— )
Catalog No. M23315 CAS No. 1121-25-1
3-Hydroxy-2-methylpyridine could as a promising molecular scaffold for the future development of novel fibrillization inhibitors.
Purity : >98% (HPLC)
 COA
COA
 Datasheet
Datasheet
 HNMR
HNMR
 HPLC
HPLC
 MSDS
MSDS
 Handing Instructions
Handing Instructions
| Size | Price / USD | Stock | Quantity |
| 1 mL x 10 mM in DMSO | 28 | In Stock |


|
| 100MG | 27 | In Stock |


|
| 200MG | Get Quote | In Stock |


|
| 500MG | Get Quote | In Stock |


|
| 1G | Get Quote | In Stock |


|
Biological Information
-
Product Name3-Hydroxy-2-methylpyridine
-
NoteResearch use only, not for human use.
-
Brief Description3-Hydroxy-2-methylpyridine could as a promising molecular scaffold for the future development of novel fibrillization inhibitors.
-
Description3-Hydroxy-2-methylpyridine could as a promising molecular scaffold for the future development of novel fibrillization inhibitors.
-
In Vitro——
-
In Vivo——
-
Synonyms——
-
PathwayOthers
-
TargetOther Targets
-
RecptorOthers
-
Research Area——
-
Indication——
Chemical Information
-
CAS Number1121-25-1
-
Formula Weight109.13
-
Molecular FormulaC6H7NO
-
Purity>98% (HPLC)
-
SolubilityDMSO:10 mM
-
SMILESCC1=C(C=CC=N1)O
-
Chemical Name——
Shipping & Storage Information
-
Storage(-20℃)
-
ShippingWith Ice Pack
-
Stability≥ 2 years
Reference
molnova catalog



related products
-
Gap19 TFA
Gap19 TFA is a peptide derived from nine amino acids of Cx43 cytoplasmic ring (CL), an effective, selective connexin 43 (Cx43) half-channel blocker.
-
Brevinin - 1
Brevinin - 1
-
Big Gastrin I (human...
Big Gastrin I (human)



 Cart
Cart
 sales@molnova.com
sales@molnova.com


