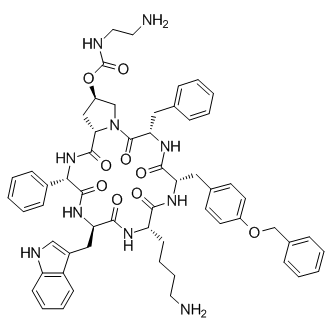
Pasireotide
CAS No. 396091-73-9
Pasireotide( SOM 230 | SOM 320 )
Catalog No. M14362 CAS No. 396091-73-9
A potent, stable cyclohexapeptide somatostatin mimic that exhibits unique high-affinity binding to human somatostatin receptors.
Purity : >98% (HPLC)
 COA
COA
 Datasheet
Datasheet
 HNMR
HNMR
 HPLC
HPLC
 MSDS
MSDS
 Handing Instructions
Handing Instructions
| Size | Price / USD | Stock | Quantity |
| 100MG | Get Quote | In Stock |


|
| 200MG | Get Quote | In Stock |


|
| 500MG | Get Quote | In Stock |


|
| 1G | Get Quote | In Stock |


|
Biological Information
-
Product NamePasireotide
-
NoteResearch use only, not for human use.
-
Brief DescriptionA potent, stable cyclohexapeptide somatostatin mimic that exhibits unique high-affinity binding to human somatostatin receptors.
-
DescriptionA potent, stable cyclohexapeptide somatostatin mimic that exhibits unique high-affinity binding to human somatostatin receptors (pKi=8.2/9.0/9.1/<7.0/9.9 for sst1/2/3/4/5, respectively); effectively inhibits GHRH-induced growth hormone release in primary cultures of rat pituitary cells with IC50 of 0.4 nM, also potently suppresses GH secretion in rats.Cushing's Disease Approved.
-
In VitroPasireotide exhibits unique high-affinity binding to human somatostatin receptors (subtypes sst1/2/3/4/5, pKi=8.2/9.0/9.1/<7.0/9.9, respectively).Pasireotide effectively inhibits the growth hormone releasing hormone (GHRH) induced growth hormone (GH) release in primary cultures of rat pituitary cells, with an IC50 of 0.4 nM.
-
In VivoPasireotide (160 mg/kg/mouth; s.c. for 4 months) significantly decreases the serum insulin, increases serum glucose, reduces the tumor size and increases apoptosis in Pdx1-Cre.Pasireotide (2-50 μg/kg; s.c. twice daily for 42 days) exerts the antinociceptive and antiinflammatory actions via the SSTR2 receptor in a mouse model of immune-mediated arthritis. Animal Model:12 month-old conditional Men1 knockout mice with insulinoma Dosage:160 mg/kg/mouth Administration:S.c. every month for 4 monthsResult:Decreased the serum insulin from 1.060 μg/L to 0.3653 μg/L and increased the serum glucose from 4.246 mM to 7.122 mM.Significantly reduced the tumor size and increased apoptosis.
-
SynonymsSOM 230 | SOM 320
-
PathwayGPCR/G Protein
-
TargetSomatostatin Receptor
-
RecptorSomatostatin Receptor
-
Research AreaEndocrinology
-
IndicationCushing Disease
Chemical Information
-
CAS Number396091-73-9
-
Formula Weight1047.206
-
Molecular FormulaC58H66N10O9
-
Purity>98% (HPLC)
-
Solubility10 mM in DMSO
-
SMILESC1C(CN2C1C(=O)NC(C(=O)NC(C(=O)NC(C(=O)NC(C(=O)NC(C2=O)CC3=CC=CC=C3)CC4=CC=C(C=C4)OCC5=CC=CC=C5)CCCCN)CC6=CNC7=CC=CC=C76)C8=CC=CC=C8)OC(=O)NCCN
-
Chemical NameCyclo[(2S)-2-phenylglycyl-D-tryptophyl-L-lysyl-O-(phenylmethyl)-L-tyrosyl-L-phenylalanyl-(4R)-4-[[[(2-aminoethyl)amino]carbonyl]oxy]-L-prolyl]
Shipping & Storage Information
-
Storage(-20℃)
-
ShippingWith Ice Pack
-
Stability≥ 2 years
Reference
1. Lewis I, et al. J Med Chem. 2003 Jun 5;46(12):2334-44.
2. Bruns C, et al. Eur J Endocrinol. 2002 May;146(5):707-16.
3. Hofland LJ, et al. Eur J Endocrinol. 2005 Apr;152(4):645-54.
molnova catalog



related products
-
Octreotide acetate
An octapeptide that mimics natural somatostatin pharmacologically, that is 200 times more potent than somatostatin.
-
Somatostatin-28 1-14
Somatostatin-28 (1-14) is an N-terminal fragment of the neuropeptide somatostatin-28. Somatostatin 28 arises from the posttranslational cleavage of prosomatostatin, which in turn is derived from a large precursor, preprosomatostatin.
-
CYN 154806 TFA
CYN 154806 TFA is a cyclic octapeptide and an effective selective somatostatin sst2 receptor antagonist. For the recombinant sst2, sst1, sst3, sst4 and sst5 receptors, the pIC50 values are 8.58, 5.41, 6.07, 5.76 and 6.48, respectively.



 Cart
Cart
 sales@molnova.com
sales@molnova.com


