WZ4002
CAS No. 1213269-23-8
WZ4002( WZ4002 | WZ-4002 | WZ 4002 )
Catalog No. M10796 CAS No. 1213269-23-8
WZ4002 is a novel, mutant-selective EGFR inhibitor for EGFR(L858R)/(T790M) with IC50 of 2 nM/8 nM.
Purity : >98% (HPLC)
 COA
COA
 Datasheet
Datasheet
 HNMR
HNMR
 HPLC
HPLC
 MSDS
MSDS
 Handing Instructions
Handing Instructions
| Size | Price / USD | Stock | Quantity |
| 1 mL x 10 mM in DMSO | 46 | In Stock |


|
| 2MG | 29 | In Stock |


|
| 5MG | 42 | In Stock |


|
| 10MG | 64 | In Stock |


|
| 25MG | 113 | In Stock |


|
| 50MG | 183 | In Stock |


|
| 100MG | 323 | In Stock |


|
| 200MG | 479 | In Stock |


|
| 500MG | Get Quote | In Stock |


|
| 1G | Get Quote | In Stock |


|
Biological Information
-
Product NameWZ4002
-
NoteResearch use only, not for human use.
-
Brief DescriptionWZ4002 is a novel, mutant-selective EGFR inhibitor for EGFR(L858R)/(T790M) with IC50 of 2 nM/8 nM.
-
DescriptionWZ4002 is a novel, mutant-selective EGFR inhibitor for EGFR(L858R)/(T790M) with IC50 of 2 nM/8 nM; does not inhibit ERBB2 phosphorylation (T798I).(In Vitro):WZ4002 increases cellular potency correlated with inhibition of EGFR, AKT and ERK1/2 phosphorylation in NSCLC cell lines and EGFR phosphorylation in NIH-3T3 cells expressing different EGFRT790M mutant alleles. WZ4002 inhibits EGFR kinase activity of recombinant L858R/T790M protein more potently than of WT EGFR.(In Vivo):In a pharmacodynamic study WZ4002 effectively inhibits EGFR, AKT and ERK1/2 phosphorylation which is associated with a significant increase in TUNEL positive and a significant decrease in Ki67 positive cells compared to vehicle alone treated mice. In a 2 week efficacy study, WZ4002 treatment results in significant tumor regressions compared to vehicle alone in both T790M containing murine models. Histological evaluation of the lungs following treatment confirms significant resolution of the tumor nodules with only few small residual nodules and nodule remnants that has evidence of treatment effect with decreased cellularity and increased fibrosis consistent with remodeling/scarring.
-
In Vitro——
-
In VivoIn a pharmacodynamic study WZ4002 effectively inhibits EGFR, AKT and ERK1/2 phosphorylation which is associated with a significant increase in TUNEL positive and a significant decrease in Ki67 positive cells compared to vehicle alone treated mice. In a 2 week efficacy study, WZ4002 treatment results in significant tumor regressions compared to vehicle alone in both T790M containing murine models. Histological evaluation of the lungs following treatment confirms significant resolution of the tumor nodules with only few small residual nodules and nodule remnants that has evidence of treatment effect with decreased cellularity and increased fibrosis consistent with remodeling/scarring.
-
SynonymsWZ4002 | WZ-4002 | WZ 4002
-
PathwayAngiogenesis
-
TargetEGFR
-
RecptorEGFR (L858R)| EGFR (L858R/T790M)
-
Research AreaCancer
-
Indication——
Chemical Information
-
CAS Number1213269-23-8
-
Formula Weight494.18
-
Molecular FormulaC25H27ClN6O3
-
Purity>98% (HPLC)
-
SolubilityDMSO:13 mg/mL (26.3 mM);Ethanol:<1 mg/mL (<1 mM);Water:<1 mg/mL (<1 mM)
-
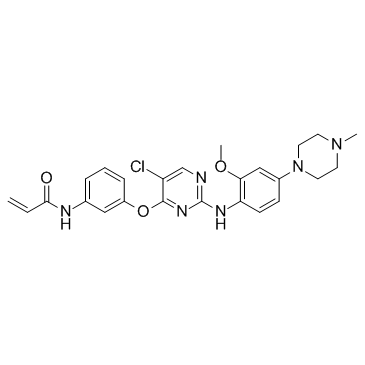
SMILESC=CC(NC1=CC=CC(OC2=NC(NC3=CC=C(N4CCN(C)CC4)C=C3OC)=NC=C2Cl)=C1)=O
-
Chemical NameN-(3-((5-chloro-2-((2-methoxy-4-(4-methylpiperazin-1-yl)phenyl)amino)pyrimidin-4-yl)oxy)phenyl)acrylamide
Shipping & Storage Information
-
Storage(-20℃)
-
ShippingWith Ice Pack
-
Stability≥ 2 years
Reference
1.Zhou W, et al. Nature. 2009, 462(7276), 1070-1074.
molnova catalog



related products
-
PD153035 hydrochlori...
PD153035 hydrochloride (ZM 252868; AG 1517) is a potent and specific inhibitor of EGFR with Ki and IC50 of 5.2 pM and 29 pM; little effect against PGDFR, FGFR, CSF-1, InsR and Src.
-
Mutated EGFR-IN-1
Mutated EGFR-IN-1 is a useful intermediate for the inhibitors design for mutated EGFR, such as L858R EGFR, Exonl9 deletion activating mutant, and T790M resistance mutant.
-
AEE-788
A potent, orally bioavailable dual EGFR/VEGFR receptor tyrosine kinase inhibitor with IC50 of 2, 6, 77 and 59 nM for EGFR, ErbB2, KDR and Flt-1, respectively.



 Cart
Cart
 sales@molnova.com
sales@molnova.com


