Lirimilast
CAS No. 329306-27-6
Lirimilast ( BAY 19-8004;BAY 198004 )
Catalog No. M14085 CAS No. 329306-27-6
Lirimilast (BAY 19-8004;BAY 198004) is a potent and selective PDE4 inhibitor for the treatment of COPD.
Purity : >98% (HPLC)
 COA
COA
 Datasheet
Datasheet
 HNMR
HNMR
 HPLC
HPLC
 MSDS
MSDS
 Handing Instructions
Handing Instructions
| Size | Price / USD | Stock | Quantity |
| 5MG | 419 | Get Quote |


|
| 50MG | 1782 | Get Quote |


|
| 100MG | 2250 | Get Quote |


|
| 200MG | Get Quote | Get Quote |


|
| 500MG | Get Quote | Get Quote |


|
| 1G | Get Quote | Get Quote |


|
Biological Information
-
Product NameLirimilast
-
NoteResearch use only, not for human use.
-
Brief DescriptionLirimilast (BAY 19-8004;BAY 198004) is a potent and selective PDE4 inhibitor for the treatment of COPD.
-
DescriptionLirimilast (BAY 19-8004;BAY 198004) is a potent and selective PDE4 inhibitor for the treatment of COPD.COPD Phase 2 Discontinued
-
SynonymsBAY 19-8004;BAY 198004
-
PathwayAngiogenesis
-
TargetPDE
-
RecptorPDE
-
Research AreaInflammation/Immunology
-
IndicationCOPD
Chemical Information
-
CAS Number329306-27-6
-
Formula Weight443.25
-
Molecular FormulaC17H12Cl2N2O6S
-
Purity>98% (HPLC)
-
Solubility——
-
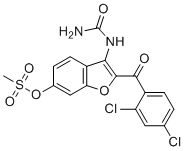
SMILESCS(=O)(OC1=CC=C2C(NC(N)=O)=C(C(C3=CC=C(Cl)C=C3Cl)=O)OC2=C1)=O
-
Chemical Name[3-(carbamoylamino)-2-(2,4-dichlorobenzoyl)-1-benzofuran-6-yl] methanesulfonate
Shipping & Storage Information
-
Storage(-20℃)
-
ShippingWith Ice Pack
-
Stability≥ 2 years
Reference
1. Grootendorst DC, et al. Pulm Pharmacol Ther. 2003;16(6):341-7.
molnova catalog


related products
-
Drotaverine hydrochl...
Drotaverine hydrochloride is a type 4 cyclic nucleotide phosphodiesterase (PDE4) inhibitor,and is an antispasmodic drug, used to enhance cervical dilation during childbirth.?
-
AZD1283
AZD1283 is an effective P2Y12 receptor antagonist (EC50: 3.0 ug/kg/min, binding IC50: 11 nM).
-
Mirodenafil
Mirodenafil is a PDE-5 inhibitor developed for the treatment of erectile dysfunction.The pharmacoki-netics of mirodenafil were not significantly altered by this concurrent administration. Mirodenafil (50 or 100 mg), obviously improved erectile function and was well tolerated in a representative population of Korean men with broad-spectrum ED of various etiologies and severities.



 Cart
Cart
 sales@molnova.com
sales@molnova.com


