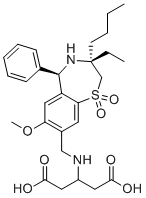
Linerixibat
CAS No. 1345982-69-5
Linerixibat( GSK-2330672 | GSK2330672 )
Catalog No. M11389 CAS No. 1345982-69-5
A highly potent, selective, ileal apical sodium-dependent bile acid transporter (ASBT) inhibitor with IC50 of 2.1/1.9 nM for mouse/rat ASBT, respectively.
Purity : >98% (HPLC)
 COA
COA
 Datasheet
Datasheet
 HNMR
HNMR
 HPLC
HPLC
 MSDS
MSDS
 Handing Instructions
Handing Instructions
| Size | Price / USD | Stock | Quantity |
| 2MG | 107 | In Stock |


|
| 5MG | 200 | In Stock |


|
| 10MG | 335 | In Stock |


|
| 25MG | 566 | In Stock |


|
| 50MG | 806 | In Stock |


|
| 100MG | Get Quote | In Stock |


|
| 200MG | Get Quote | In Stock |


|
| 500MG | Get Quote | In Stock |


|
| 1G | Get Quote | In Stock |


|
Biological Information
-
Product NameLinerixibat
-
NoteResearch use only, not for human use.
-
Brief DescriptionA highly potent, selective, ileal apical sodium-dependent bile acid transporter (ASBT) inhibitor with IC50 of 2.1/1.9 nM for mouse/rat ASBT, respectively.
-
DescriptionA highly potent, selective, ileal apical sodium-dependent bile acid transporter (ASBT) inhibitor with IC50 of 2.1/1.9 nM for mouse/rat ASBT, respectively; lowers glucose in an animal model of type 2 diabetes, shows excellent developability properties for evaluating the potential therapeutic utility of a nonabsorbable ASBT inhibitor for treatment of patients with type 2 diabetes.Diabetes Phase 2 Clinical(In Vitro):The zwitterionic, nonhygroscopic, crystalline salt form of Linerixibat (Compound 56) shows good aqueous solubility at pH 7.4 (>7 mg/mL), excellent thermal stability, and did not generate reactive or humanspecific metabolite, characteristics.(In Vivo):Linerixibat (GSK2330672; 0.05-10 mg/kg; oral gavage; twice daily; for 14 days; male ZDF rat) treatment lowers glucose in an animal model of type 2 diabetes.
-
In Vitro——
-
In VivoAnimal Model:Male Zucker Diabetic Fatty (ZDF) rat Dosage:0.05 mg/kg, 0.1 mg/kg, 0.5 mg/kg, 1 mg/kg, 5 mg/kg, 10 mg/kg Administration:Oral gavage; twice daily; for 14 days Result:Led to a 1.30-1.64% reduction in hemoglobin A1c (HbA1c), a greater than 50% reduction in nonfasted plasma glucose to below 200 mg/dL, and statistically significant higher plasma insulin.
-
SynonymsGSK-2330672 | GSK2330672
-
PathwayMembrane Transporter/Ion Channel
-
TargetASBT Transporter
-
RecptorhASBT
-
Research AreaMetabolic Disease
-
IndicationDiabetes
Chemical Information
-
CAS Number1345982-69-5
-
Formula Weight546.679
-
Molecular FormulaC28H38N2O7S
-
Purity>98% (HPLC)
-
SolubilityDMSO: 21.5 mg/mL ( < 1 mg/ml refers to the product slightly soluble or insoluble )
-
SMILESO=C(O)CC(NCC1=C(OC)C=C(C2=C1)[C@@H](C3=CC=CC=C3)N[C@](CC)(CCCC)CS2(=O)=O)CC(O)=O
-
Chemical Name3-[[[(3R,5R)-3-Butyl-3-ethyl-2,3,4,5-tetrahydro-7-methoxy-1,1-dioxido-5-phenyl-1,4-benzothiazepin-8-yl]methyl]amino]pentanedioic acid
Shipping & Storage Information
-
Storage(-20℃)
-
ShippingWith Ice Pack
-
Stability≥ 2 years
Reference
1. Wu Y, et al. J Med Chem. 2013 Jun 27;56(12):5094-114.
2. Nunez DJ, et al. Diabetes Obes Metab. 2016 Jul;18(7):654-62.
3. Hegade VS, et al. Lancet. 2017 Mar 18;389(10074):1114-1123.
molnova catalog



related products
-
Volixibat potassium
A highly potent and selective inhibitor of the apical sodium-dependent bile acid transporter (ASBT) in development for the treatment of nonalcoholic steatohepatitis (NASH).
-
Elobixibat
An orally available, potent and selective ileal bile acid transporter (IBAT) inhibitor for the treatment of constipation.
-
Lopixibat chloride
Lopixibat chloride (LUM-001, HP-625) is a potent, selectiove, oral inhibitor of sodium bile acid cotransporter and Ileal bile acid transporter (IBAT).



 Cart
Cart
 sales@molnova.com
sales@molnova.com


