LY500307
CAS No. 533884-09-2
LY500307( LY-500307 | LY 500307 | Erteberel | SERBA-1 )
Catalog No. M14897 CAS No. 533884-09-2
LY500307 (LY-500307, Erteberel, SERBA-1) is a potent, selective Estrogen Receptor β (ERβ) agonist with Ki/EC50 of 0.19/0.66 nM.
Purity : >98% (HPLC)
 COA
COA
 Datasheet
Datasheet
 HNMR
HNMR
 HPLC
HPLC
 MSDS
MSDS
 Handing Instructions
Handing Instructions
| Size | Price / USD | Stock | Quantity |
| 2MG | 215 | Get Quote |


|
| 5MG | 410 | Get Quote |


|
| 10MG | 591 | Get Quote |


|
| 25MG | 888 | Get Quote |


|
| 100MG | Get Quote | Get Quote |


|
| 200MG | Get Quote | Get Quote |


|
| 500MG | Get Quote | Get Quote |


|
| 1G | Get Quote | Get Quote |


|
Biological Information
-
Product NameLY500307
-
NoteResearch use only, not for human use.
-
Brief DescriptionLY500307 (LY-500307, Erteberel, SERBA-1) is a potent, selective Estrogen Receptor β (ERβ) agonist with Ki/EC50 of 0.19/0.66 nM.
-
DescriptionLY500307 (LY-500307, Erteberel, SERBA-1) is a potent, selective Estrogen Receptor β (ERβ) agonist with Ki/EC50 of 0.19/0.66 nM, 12-fold higher affinity than ERα and exhibits 32-fold more functional potency; reduces proliferation and induces apoptosis in GBM cells, enhances ERβ signaling and expression, modulates the expression of genes involved in cell cycle, cell death, survival, and DNA damage response; also sensitizes GBM cells to chemotherapeutic agents, reduces GBM progression in an orthotopic model .Schizophrenia Phase 2 Clinical.
-
In VitroTreatment with Erteberel (0.25-10 μM, 72 hours) significantly reduces the proliferation of GBM cells with no activity on normal astrocytes in vitro. Erteberel promotes apoptosis of GBM cells. Erteberel modulated several pathways related to apoptosis, cell cycle, and DNA damage response. Erteberel (0-1000 μM) sensitizes GBM cells to several FDA-approved chemotherapeutic drugs including cisplatin, lomustine and temozolomide.Cell Viability Assay Cell Line:U87, U251,T98G and normal astrocytesConcentration:0.25, 0.5, 1, 2, 4, 6, 8, and 10 μMIncubation Time:72 h Result:Treatment with Erteberel significantly reduces the viability of various GBM cell lines in a dose-dependent manner. In contrast, viability of normal astrocytes is not affected at the tested doses, suggesting that Erteberel has tumor cell–specific activity.
-
In VivoErteberel (5?mg/Kg body weight/day, oral, 28 days) treatment significantly reduces tumor growth and promotes apoptosis of GBM tumors in an orthotopic model.Erteberel (5?mg/Kg body weight/day, oral, 40-50 days) treatment improves the overall survival of tumor-bearing mice in the GL26 syngeneic glioma model. Animal Model:Athymic mice (5-7 weeks) inoculated with OVCAR-3 cells Dosage:5mg/Kg body weight Administration:Oral, daily for 28 days Result:Immunohistochemical analysis reveals that Erteberel treatment significantly reduces the number of proliferation marker Ki-67-positive cells and increases the number of TUNEL-positive apoptotic cells.
-
SynonymsLY-500307 | LY 500307 | Erteberel | SERBA-1
-
PathwayEndocrinology/Hormones
-
TargetEstrogen Receptor/ERR
-
RecptorERβ
-
Research AreaNeurological Disease
-
IndicationSchizophrenia
Chemical Information
-
CAS Number533884-09-2
-
Formula Weight282.339
-
Molecular FormulaC18H18O3
-
Purity>98% (HPLC)
-
SolubilityIn Vitro:?DMSO : ≥ 30 mg/mL (106.26 mM)
-
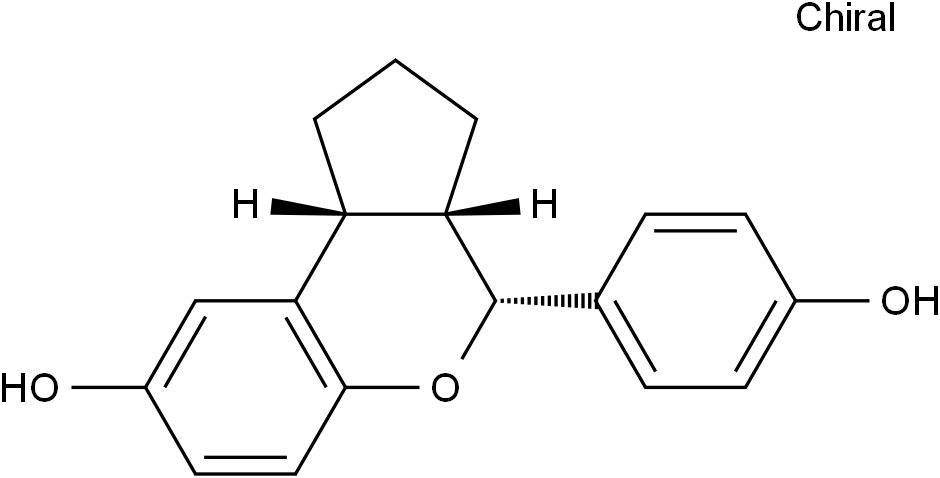
SMILESOC1=CC=C2C([C@@](CCC3)([H])[C@@]3([H])[C@H](C4=CC=C(O)C=C4)O2)=C1
-
Chemical Name(3aS,4R,9bR)-1,2,3,3a,4,9b-Hexahydro-4-(4-hydroxyphenyl)cyclopenta[c][1]benzopyran-8-ol
Shipping & Storage Information
-
Storage(-20℃)
-
ShippingWith Ice Pack
-
Stability≥ 2 years
Reference
1. Sareddy GR, et al. Sci Rep. 2016 Apr 29;6:24185.
2. Norman BH, et al.
3. Zhao L, et al. Proc Natl Acad Sci U S A. 2018 Apr 17;115(16):E3673-E3681.
4. Reese JM, et al. Oncotarget. 2017 Oct 11;8(57):96506-96521.
molnova catalog



related products
-
LSZ102
LSZ102 is a potent, orally bioavailable, selective estrogen receptor degrader (SERD) with ERα transcription IC50 of 6 nM and ERα degradation IC50 of 0.2 nM.
-
PHTPP
A potent, selective antagonist of estrogen ERβ receptor; exhibits 36-fold selective for ERβ, and is fully effective as an ERα antagonist.
-
Quinestrol
Quinestrol? is a synthetic estrogen, used in hormone replacement therapy, and occasionally to treat breast cancer and prostate cancer.



 Cart
Cart
 sales@molnova.com
sales@molnova.com


