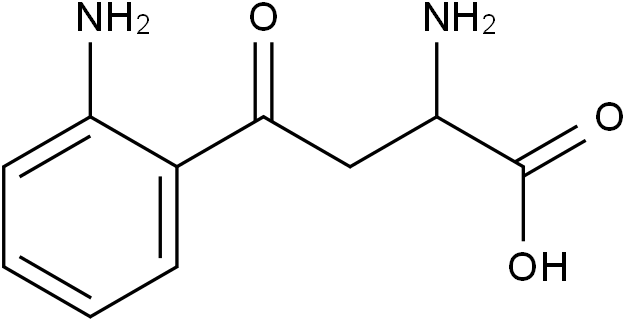
Kynurenine
CAS No. 343-65-7
Kynurenine( Kynurenine )
Catalog No. M14171 CAS No. 343-65-7
Kynurenine is synthesized by the enzyme tryptophan dioxygenase, which is made primarily but not exclusively in the liver.
Purity : >98% (HPLC)
 COA
COA
 Datasheet
Datasheet
 HNMR
HNMR
 HPLC
HPLC
 MSDS
MSDS
 Handing Instructions
Handing Instructions
| Size | Price / USD | Stock | Quantity |
| 25MG | 35 | In Stock |


|
| 50MG | 53 | In Stock |


|
| 100MG | Get Quote | In Stock |


|
| 200MG | Get Quote | In Stock |


|
| 500MG | Get Quote | In Stock |


|
| 1G | Get Quote | In Stock |


|
Biological Information
-
Product NameKynurenine
-
NoteResearch use only, not for human use.
-
Brief DescriptionKynurenine is synthesized by the enzyme tryptophan dioxygenase, which is made primarily but not exclusively in the liver.
-
DescriptionKynurenine is synthesized by the enzyme tryptophan dioxygenase, which is made primarily but not exclusively in the liver, and indoleamine 2,3-dioxygenase, which is made in many tissues in response to immune activation.
-
In Vitro——
-
In Vivo——
-
SynonymsKynurenine
-
PathwayOthers
-
TargetOther Targets
-
RecptorOthers
-
Research Area——
-
Indication——
Chemical Information
-
CAS Number343-65-7
-
Formula Weight208.22
-
Molecular FormulaC10H12N2O3
-
Purity>98% (HPLC)
-
SolubilityDMSO: 10 mM
-
SMILESO=C(O)C(N)CC(C1=CC=CC=C1N)=O
-
Chemical Namealpha-2-Diamino-gamma-oxobenzenebutyric acid
Shipping & Storage Information
-
Storage(-20℃)
-
ShippingWith Ice Pack
-
Stability≥ 2 years
Reference
1. Liu JJ, et al. Biochim Biophys Acta. 2015 May;1852(5):980-91.
molnova catalog



related products
-
Epigambogic acid
Epigambogic acid is a natural compound that can be extracted from plants such as cotton root and sweetgum.
-
ML390
ML390 exerts its potent differentiation effect on multiple leukemia models.
-
Biotin-4-aminophenol
Biotin-4-aminophenol (BP) is a selective and potent biotin-phenol analog that works by generating free radicals and binding to tyrosine residues in proteins.



 Cart
Cart
 sales@molnova.com
sales@molnova.com


