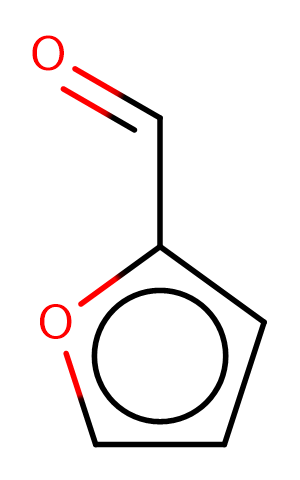
Furfural
CAS No. 98-01-1
Furfural( furan-2-carboxaldehyde | 2-furaldehyde | pyromucic aldehyde | furfuraldehyde )
Catalog No. M20306 CAS No. 98-01-1
Furfural is an aldehyde that is?furan?with the?hydrogen?at position 2 substituted by a formyl group. It has a role as a Maillard reaction product and a metabolite.
Purity : >98% (HPLC)
 COA
COA
 Datasheet
Datasheet
 HNMR
HNMR
 HPLC
HPLC
 MSDS
MSDS
 Handing Instructions
Handing Instructions
| Size | Price / USD | Stock | Quantity |
| 100MG | Get Quote | Get Quote |


|
| 200MG | Get Quote | Get Quote |


|
| 500MG | Get Quote | Get Quote |


|
| 1G | Get Quote | Get Quote |


|
Biological Information
-
Product NameFurfural
-
NoteResearch use only, not for human use.
-
Brief DescriptionFurfural is an aldehyde that is?furan?with the?hydrogen?at position 2 substituted by a formyl group. It has a role as a Maillard reaction product and a metabolite.
-
DescriptionFurfural is an aldehyde that is?furan?with the?hydrogen?at position 2 substituted by a formyl group. It has a role as a Maillard reaction product and a metabolite.
-
In Vitro——
-
In Vivo——
-
Synonymsfuran-2-carboxaldehyde | 2-furaldehyde | pyromucic aldehyde | furfuraldehyde
-
PathwayOthers
-
TargetOther Targets
-
RecptorOthers
-
Research Area——
-
Indication——
Chemical Information
-
CAS Number98-01-1
-
Formula Weight96.08
-
Molecular FormulaC5H4O2
-
Purity>98% (HPLC)
-
Solubility——
-
SMILESO=Cc1ccco1
-
Chemical Name——
Shipping & Storage Information
-
Storage(-20℃)
-
ShippingWith Ice Pack
-
Stability≥ 2 years
Reference
1.Enhanced stabilization of collagen by furfural[J]. International Journal of Biological Macromolecules 2014 65:252-257.
molnova catalog



related products
-
Chlorpropham
Chlorpropham acts as a plant growth regulator and herbicide.
-
Sematilide HCl
Sematilide (CK-1752) is a class III antiarrhythmic. It is a selectively delayed rectifier K+ current (IKr) channel blocker.
-
Memantine
Memantine, an amantadine derivative with some dopaminergic effects, has been proposed as an antiparkinson agent and has may be used to treat moderate to severe Alzheimer's disease. It acts on the glutamatergic system by blocking NMDA receptors.



 Cart
Cart
 sales@molnova.com
sales@molnova.com


