Cadazolid
CAS No. 1025097-10-2
Cadazolid( ACT-179811 | Cadazolid. )
Catalog No. M17120 CAS No. 1025097-10-2
Cadazolid is an oxazolidinone-type antibiotic, with activity against gram-positive bacteria, including Clostridium difficile.
Purity : >98% (HPLC)
 COA
COA
 Datasheet
Datasheet
 HNMR
HNMR
 HPLC
HPLC
 MSDS
MSDS
 Handing Instructions
Handing Instructions
| Size | Price / USD | Stock | Quantity |
| 2MG | 40 | In Stock |


|
| 5MG | 65 | In Stock |


|
| 10MG | 110 | In Stock |


|
| 25MG | 200 | In Stock |


|
| 50MG | 332 | In Stock |


|
| 100MG | 494 | In Stock |


|
| 200MG | Get Quote | In Stock |


|
| 500MG | Get Quote | In Stock |


|
| 1G | Get Quote | In Stock |


|
Biological Information
-
Product NameCadazolid
-
NoteResearch use only, not for human use.
-
Brief DescriptionCadazolid is an oxazolidinone-type antibiotic, with activity against gram-positive bacteria, including Clostridium difficile.
-
DescriptionCadazolid, also known ACT-179811, is a novel fluoroquinolone-oxazolidinone antibiotic and a protein synthesis inhibitor. Cadazolid may be potentially useful for the treatment of Clostridium difficile infection. Cadazolid exhibits potent in vitro activity against Clostridium difficile, including the epidemic BI/NAP1/027 strain. Clostridium difficile infection (CDI), the main cause of nosocomial infectious diarrhea, results from the growth of toxin-producing C. difficile in the colon following disruption of the normal enteric microbiota, usually as a consequence of antibiotic therapy.(In Vitro):Cadazolid is a new antibiotic in development for the treatment of Clostridium difficile-associated diarrhea. Cadazolid is active against all (including linezolid- and moxifloxacin-resistant) Clostridium difficile strains (MIC90 0.125, range 0.03-0.25 mg/L). The cadazolid geometric mean MIC is 152-fold, 16-fold, 9-fold and 7-fold lower than those of moxifloxacin, linezolid, metronidazole and vancomycin, respectively. Both cadazolid dosing regimens rapidly reduce Clostridium difficile viable counts and cytotoxin with no evidence of recurrence. Cadazolid levels persists at 50-100-fold supra-MIC for 14 days post-dosing. Cadazolid inhibition of enumerated gut microflora is limited, with the exception of bifidobacteria; Bacteroides fragilis group and Lactobacillus spp. counts are unaffected. There is no evidence for selection of strains resistant to cadazolid, quinolones or linezolid. (In Vivo):Cadazolid is well tolerated up to 3000 mg given twice daily for 10 days. The most common adverse event is headache, with no observed relationship between dose or treatment duration and adverse events. Plasma concentrations of cadazolid are low. No plasma concentrations >3.3 ng/mL are observed after single doses or >6.9 ng/mL after 10 days of multiple doses. Food increased the mean Cmax from 0.73 to 1.87 ng/mL and mean AUC0–t from 3.13 to 15.69 ng·h/mL after a single 300 mg dose. The increase in systemic exposure to cadazolid across doses is less than dose-proportional. The mean cumulative faecal recovery is 81.0%–93.5%. Urinary recovery of unchanged compound is less than 0.015%.
-
In VitroCadazolid is a new antibiotic in development for the treatment of Clostridium difficile-associated diarrhea. Cadazolid is active against all (including linezolid- and moxifloxacin-resistant) Clostridium difficile strains (MIC90 0.125, range 0.03-0.25 mg/L). The cadazolid geometric mean MIC is 152-fold, 16-fold, 9-fold and 7-fold lower than those of moxifloxacin, linezolid, metronidazole and vancomycin, respectively. Both cadazolid dosing regimens rapidly reduce Clostridium difficile viable counts and cytotoxin with no evidence of recurrence. Cadazolid levels persists at 50-100-fold supra-MIC for 14 days post-dosing. Cadazolid inhibition of enumerated gut microflora is limited, with the exception of bifidobacteria; Bacteroides fragilis group and Lactobacillus spp. counts are unaffected. There is no evidence for selection of strains resistant to cadazolid, quinolones or linezolid.
-
In VivoCadazolid is well tolerated up to 3000 mg given twice daily for 10 days. The most common adverse event is headache, with no observed relationship between dose or treatment duration and adverse events. Plasma concentrations of cadazolid are low. No plasma concentrations >3.3 ng/mL are observed after single doses or >6.9 ng/mL after 10 days of multiple doses. Food increased the mean Cmax from 0.73 to 1.87 ng/mL and mean AUC0–t from 3.13 to 15.69 ng·h/mL after a single 300 mg dose. The increase in systemic exposure to cadazolid across doses is less than dose-proportional. The mean cumulative faecal recovery is 81.0%–93.5%. Urinary recovery of unchanged compound is less than 0.015%.
-
SynonymsACT-179811 | Cadazolid.
-
PathwayOthers
-
TargetOther Targets
-
RecptorOthers
-
Research Area——
-
Indication——
Chemical Information
-
CAS Number1025097-10-2
-
Formula Weight585.19
-
Molecular FormulaC29H29F2N3O8
-
Purity>98% (HPLC)
-
SolubilityDMSO : ≥ 150 mg/mL 256.17 mM
-
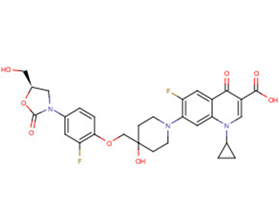
SMILESc1c(c(cc2c1n(cc(c2=O)C(=O)O)C1CC1)F)N1CCC(CC1)(COc1c(cc(cc1)N1C[C@@H](OC1=O)CO)F)O
-
Chemical Name1-Cyclopropyl-6-fluoro-7-[4-({2-fluoro-4-[(5R)-5-(hydroxymethyl)-2-oxo-1,3-oxazolidin-3-yl]phenoxy}methyl)-4-hydroxypiperidin-1-yl]-4-oxo-1,4-dihydroquinolin-3-carboxylic acid
Shipping & Storage Information
-
Storage(-20℃)
-
ShippingWith Ice Pack
-
Stability≥ 2 years
Reference
1. Seiler P., et al. Cadazolid does not promote intestinal colonization of vancomycin-resistant enterococci in mice. Antimicrob Agents Chemother. 2015 Oct 26;60(1):628-31.
molnova catalog



related products
-
POPS
POPS is a useful organic compound for research related to life sciences. The catalog number is TF0061 and the CAS number is 321863-21-2.
-
Panasenoside
Panasenoside is a natural product.
-
[Leu5]-Enkephalin, a...
[Leu5]-Enkephalin, amide is a δ opioid receptor agonist. Enkephalin, one of several naturally occurring morphinelike substances (endorphins) released from nerve endings of the central nervous system and the adrenal medulla.



 Cart
Cart
 sales@molnova.com
sales@molnova.com


