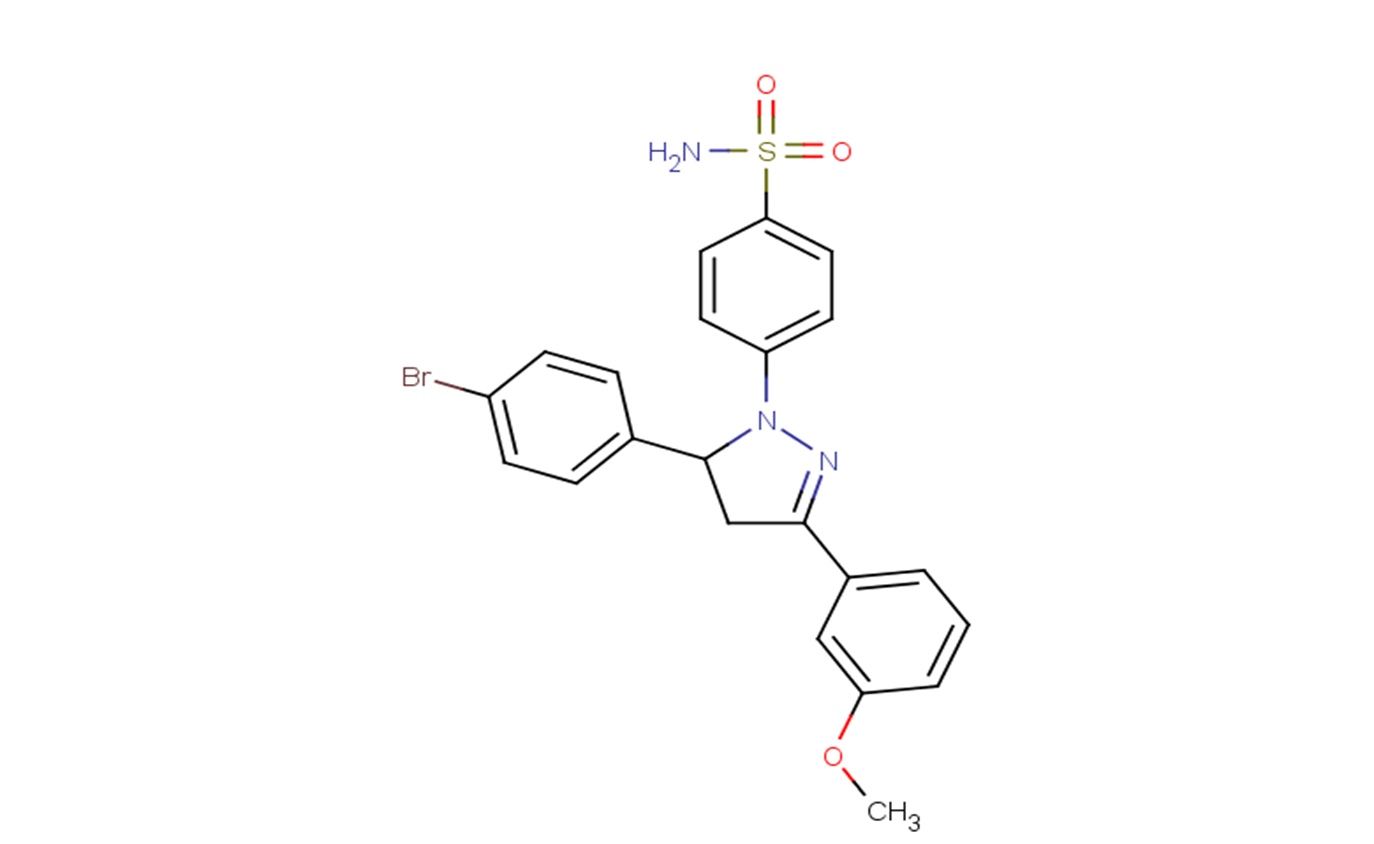
CID44216842
CAS No. 1222513-26-9
CID44216842( KUC103479N-02 )
Catalog No. M23391 CAS No. 1222513-26-9
CID44216842 is a potent Cdc42-selective guanine nucleotide binding lead inhibitor.?
Purity : >98% (HPLC)
 COA
COA
 Datasheet
Datasheet
 HNMR
HNMR
 HPLC
HPLC
 MSDS
MSDS
 Handing Instructions
Handing Instructions
| Size | Price / USD | Stock | Quantity |
| 2MG | 43 | In Stock |


|
| 5MG | 72 | In Stock |


|
| 10MG | 123 | In Stock |


|
| 25MG | 255 | In Stock |


|
| 50MG | 407 | In Stock |


|
| 100MG | 599 | In Stock |


|
| 200MG | Get Quote | In Stock |


|
| 500MG | Get Quote | In Stock |


|
| 1G | Get Quote | In Stock |


|
Biological Information
-
Product NameCID44216842
-
NoteResearch use only, not for human use.
-
Brief DescriptionCID44216842 is a potent Cdc42-selective guanine nucleotide binding lead inhibitor.?
-
DescriptionCID44216842 is a potent Cdc42-selective guanine nucleotide binding lead inhibitor.?The EC50s for Cdc42 WT and Cdc42Q61L mutant are 1.0 and 1.2 μM in GTP binding assay, respectively.?The EC50s for Cdc42 WT and Cdc42Q61L mutant are 0.3 and 0.5 μM in GDP binding assay, respectively.?Use as a molecular probe.
-
In VitroCID44216842 inhibits GTP binding to both Cdc42 and its mutant in a dose-dependent manner. The inhibition is specific toward Cdc42 with no effects on other GTPases including Rac and Rho in the same family.
-
In Vivo——
-
SynonymsKUC103479N-02
-
PathwayAngiogenesis
-
TargetCDK
-
RecptorCdc42
-
Research Area——
-
Indication——
Chemical Information
-
CAS Number1222513-26-9
-
Formula Weight486.38
-
Molecular FormulaC22H20BrN3O3S
-
Purity>98% (HPLC)
-
SolubilityDMSO:250 mg/mL?(514.00 mM;?Need ultrasonic)
-
SMILESO=S(C1=CC=C(N2N=C(C3=CC=CC(OC)=C3)CC2C4=CC=C(Br)C=C4)C=C1)(N)=O
-
Chemical Name——
Shipping & Storage Information
-
Storage(-20℃)
-
ShippingWith Ice Pack
-
Stability≥ 2 years
Reference



-
CCT251545
CCT251545 is a potent, orally bioavailable inhibitor of Wnt signaling with IC50 of 5 nM, also is a potent and selective chemical probe for CDK8 and CDK19 with >100-fold selectivity over 291 other kinases; potently inhibits reporter-based readouts of basal WNT pathway activity in LS174T and SW480 cells in the absence of WNT ligand with IC50 of 23 and 190 nM, respectively.
-
Ibulocydine
Ibulocydine is the prodrug of CDK inhibitor BMK-Y101, inhibits CDK7 and CDK9 with IC50 of 530 nM and 85 nM in kinase assays, respectively.
-
LY3405105
1-Piperidinecarboxylic acid, 4-[[5-methyl-3-(1-methylethyl)pyrazolo[1,5-a]pyrimidin-7-yl]amino]-, 1-[(2E)-4-(dimethylamino)-1-oxo-2-buten-1-yl]-3-pyrrolidinyl ester is a novel CDK7 inhibitors.



 Cart
Cart
 sales@molnova.com
sales@molnova.com


