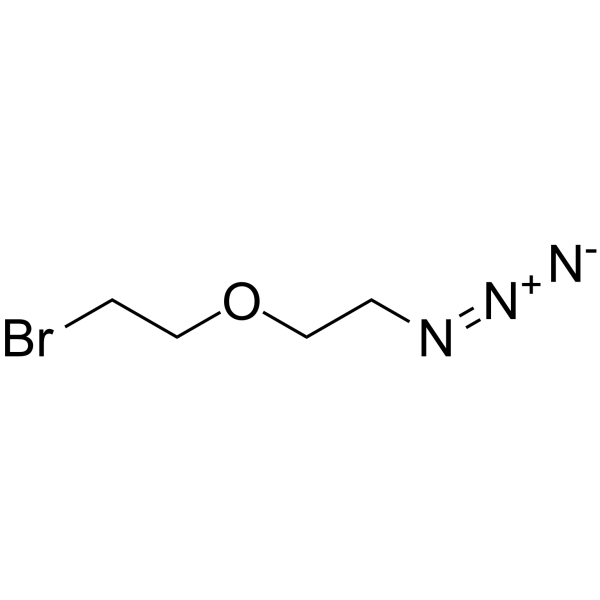
Bromo-PEG1-C2-azide
CAS No. 1144106-65-9
Bromo-PEG1-C2-azide( —— )
Catalog No. M26932 CAS No. 1144106-65-9
Bromo-PEG1-C2-azide is a PEG-based PROTAC linker that can be used in the synthesis of PROTACs.
Purity : >98% (HPLC)
 COA
COA
 Datasheet
Datasheet
 HNMR
HNMR
 HPLC
HPLC
 MSDS
MSDS
 Handing Instructions
Handing Instructions
| Size | Price / USD | Stock | Quantity |
| 5MG | 61 | In Stock |


|
| 10MG | 87 | In Stock |


|
| 25MG | 143 | In Stock |


|
| 50MG | 210 | In Stock |


|
| 100MG | 300 | In Stock |


|
| 200MG | 447 | In Stock |


|
| 500MG | 732 | In Stock |


|
| 1G | Get Quote | In Stock |


|
Biological Information
-
Product NameBromo-PEG1-C2-azide
-
NoteResearch use only, not for human use.
-
Brief DescriptionBromo-PEG1-C2-azide is a PEG-based PROTAC linker that can be used in the synthesis of PROTACs.
-
DescriptionBromo-PEG1-C2-azide is a PEG-based PROTAC linker that can be used in the synthesis of PROTACs.(In Vitro):PROTACs contain two different ligands connected by a linker; one is a ligand for an E3 ubiquitin ligase and the other is for the target protein. PROTACs exploit the intracellular ubiquitin-proteasome system to selectively degrade target proteins.
-
In VitroPROTACs contain two different ligands connected by a linker; one is a ligand for an E3 ubiquitin ligase and the other is for the target protein. PROTACs exploit the intracellular ubiquitin-proteasome system to selectively degrade target proteins.
-
In Vivo——
-
Synonyms——
-
PathwayOthers
-
TargetOther Targets
-
RecptorHuman Endogenous Metabolite
-
Research Area——
-
Indication——
Chemical Information
-
CAS Number1144106-65-9
-
Formula Weight194.032
-
Molecular FormulaC4H8BrN3O
-
Purity>98% (HPLC)
-
Solubility——
-
SMILESBrCCOCCN=[N+]=[N-]
-
Chemical Name——
Shipping & Storage Information
-
Storage(-20℃)
-
ShippingWith Ice Pack
-
Stability≥ 2 years
Reference
1.Seiler N, et al. The influence of catabolic reactions on polyamine excretion. Biochem J. 1985 Jan 1;225(1):219-26.
molnova catalog



related products
-
Neuropeptide K, porc...
Neuropeptide K, porcine
-
Urotensin II (Frog)
Urotensin II (Frog)
-
EN106
EN106 is a potent inhibitor of FEMIB and is a cysteine-reactive covalent ligand. EN106 disrupts recognition of the key reductive stress substrate of FEM1B, FNIP1.



 Cart
Cart
 sales@molnova.com
sales@molnova.com


