Amsilarotene
CAS No. 125973-56-0
Amsilarotene( TAC101 | TAC-101 | TAC 101 )
Catalog No. M27389 CAS No. 125973-56-0
Amsilarotene inhibits the phosphorylation of retinoblastoma gene product (RB) and increases the presence of 2 cyclin-dependent kinases (CDK) inhibitors resulting in cell cycle arrest.
Purity : >98% (HPLC)
 COA
COA
 Datasheet
Datasheet
 HNMR
HNMR
 HPLC
HPLC
 MSDS
MSDS
 Handing Instructions
Handing Instructions
| Size | Price / USD | Stock | Quantity |
| 5MG | 132 | In Stock |


|
| 10MG | 215 | In Stock |


|
| 25MG | 430 | In Stock |


|
| 50MG | 620 | In Stock |


|
| 100MG | 884 | In Stock |


|
| 200MG | Get Quote | In Stock |


|
| 500MG | Get Quote | In Stock |


|
| 1G | Get Quote | In Stock |


|
Biological Information
-
Product NameAmsilarotene
-
NoteResearch use only, not for human use.
-
Brief DescriptionAmsilarotene inhibits the phosphorylation of retinoblastoma gene product (RB) and increases the presence of 2 cyclin-dependent kinases (CDK) inhibitors resulting in cell cycle arrest.
-
DescriptionAmsilarotene inhibits the phosphorylation of retinoblastoma gene product (RB) and increases the presence of 2 cyclin-dependent kinases (CDK) inhibitors resulting in cell cycle arrest. This agent also causes a cytotoxic decline in the thymidylate synthase and cyclin A expression.(In Vitro):Preclinical models have shown that Amsilarotene(4-[3,5-bis(trimethylsilyl) benzamide] benzoic acid), an oral synthetic retinoid, has antitumor activity in hepatocellular carcinoma (HCC).(In Vivo):We conducted a phase I study in Japanese patients with advanced HCC to examine the pharmacokinetics, recommended dose, safety, and efficacy of Amsilarotene. The administered dose of Amsilarotene was 10 mg/day in four patients (level 1), 20 mg/day in six (level 2), and 30 mg/day in three (level 3). There was no dose-limiting toxicity at level 1. Only one patient each had dose-limiting toxicity at level 2 (grade 2 fatigue, recovery requiring eight or more consecutive days of rest) and at level 3 (grade 3 splenic vein thrombosis). Level 3 (30 mg/day) was considered the maximum tolerated dose and 20 mg/day the recommended dose by a panel of medical experts, placing maximum emphasis on safety. The most frequent adverse events were fatigue, headache, and dermal symptoms such as rash. Pharmacokinetic parameters in Japanese patients with HCC were similar to those in patients in the United States, most of whom were Caucasian. Although no patient had a complete or partial response, the disease control rate was 38.5%. In conclusion, the recommended dose of Amsilarotene for patients with HCC is 20 mg/day. Amsilarotene had an acceptable toxicity profile, warranting further evaluation in clinical trials.
-
In VitroAmsilarotene (0, 10, 25 μM; 24 hours) induces apoptosis of human epithelial ovarian carcinoma-derived cell lines in a concentration-dependent manner.Amsilarotene (10, 20 μM; 0, 3, 6, and 9 days) inhibits the proliferation of BxPC-3 and MIAPaCa-2 cells.Amsilarotene (10 μM; 48 hours) increases the proportion of sensitive BxPC-3 cells in the G1 phase.Amsilarotene (10 μM; 0, 3, 6, 24, 48, 72 hours) inhibits the retinoblastoma-gene product (RB) phosphorylation in BxPC-3 cells between 24 and 72 hours. Apoptosis Analysis Cell Line:RMG-I, RMG-II, RTSG, RMUG-S, RMUG-L, and KF cells Concentration:0, 10, 25 μM Incubation Time:24 hours Result:Induced apoptosis in a concentration-dependent manner in all of the cell lines, except KF cells.Cell Proliferation Assay Cell Line:BxPC-3, MIAPaCa-2, AsPC-1 cells Concentration:10 and 20 μM Incubation Time:0, 3, 6, and 9 days.Result:Inhibited the proliferation of BxPC-3 and MIAPaCa-2 cells, but not the proliferation of AsPC-1 cells.Cell Cycle Analysis Cell Line:Sensitive BxPC-3 cells Concentration:10 μMIncubation Time:48 hoursResult:The proportion of cells in the G1 phase increased from 43% of untreated control cells to 86%
-
In VivoAmsilarotene (8 mg/kg/day orally for 30 days) inhibits the RMG-II tumor growth in nude mice. Animal Model:6-week-old female BALB/c nu/nu mice with subcutaneous RMG-II tumors Dosage:8 mg/kg/day Administration:Orally for 30 days Result:The maximal tumor growth-inhibiting effect was seen on day 31 of administration, when there was a 45% reduction of relative tumor volume (RTV).
-
SynonymsTAC101 | TAC-101 | TAC 101
-
PathwayAngiogenesis
-
TargetCDK
-
RecptorDNA/RNA Synthesis
-
Research Area——
-
Indication——
Chemical Information
-
CAS Number125973-56-0
-
Formula Weight385.61
-
Molecular FormulaC20H27NO3Si2
-
Purity>98% (HPLC)
-
SolubilityIn Vitro:?DMSO : 100 mg/mL (259.34 mM)
-
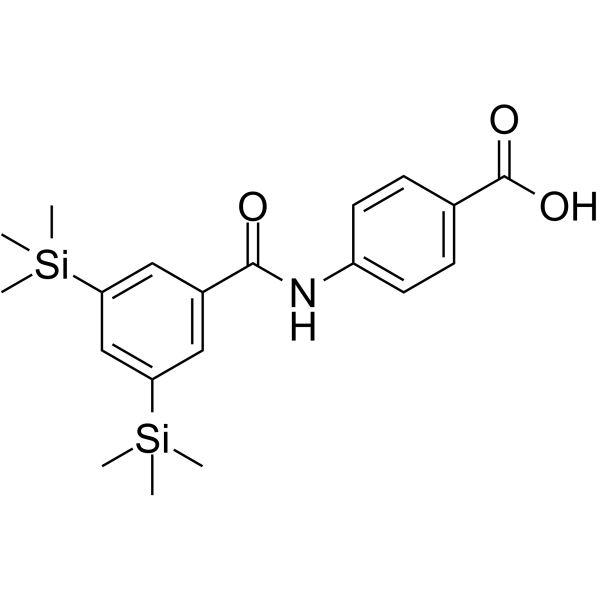
SMILESC[Si](C)(C)c1cc(cc(c1)[Si](C)(C)C)C(=O)Nc1ccc(cc1)C(O)=O
-
Chemical Name——
Shipping & Storage Information
-
Storage(-20℃)
-
ShippingWith Ice Pack
-
Stability≥ 2 years
Reference
1.Cahyani DM, Miatmoko A, Hariawan BS, Purwantari KE, Sari R. N-nitrosodiethylamine induces inflammation of liver in mice. J Basic Clin Physiol Pharmacol. 2021 Jun 25;32(4):505-510. doi: 10.1515/jbcpp-2020-0475. PMID: 34214328.
molnova catalog



related products
-
Atuveciclib Racemate
Atuveciclib (BAY-1143572) inhibits the proliferation of 7 MLL-rearrangements positive and negative AML cell lines with a median IC50 of 385 nM (range 230-1100 nM) and induces apoptosis.
-
MBQ-167
MBQ-167 is a dual inhibitor of Rac/Cdc42 (IC50s: 103 nM for Rac 1/2/3 and 78 nM for Cdc42 in MDA-MB-231 cells, respectively).
-
FMF-04-159-2
FMF-04-159-2 is a potent, selective, covalent CDK14 inhibitor (IC50=86 nM) with pan-TAIRE family specificity (CDKs 14-18).



 Cart
Cart
 sales@molnova.com
sales@molnova.com


