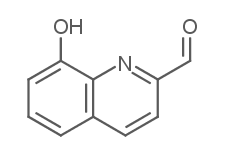
8-Hydroxyquinoline-2-carboxaldehyde
CAS No. 14510-06-6
8-Hydroxyquinoline-2-carboxaldehyde( —— )
Catalog No. M18027 CAS No. 14510-06-6
8-Hydroxyquinoline-2-carbaldehyde is a biochemical reagent that can be used as a biological material or organic compound for life science related research.
Purity : >98% (HPLC)
 COA
COA
 Datasheet
Datasheet
 HNMR
HNMR
 HPLC
HPLC
 MSDS
MSDS
 Handing Instructions
Handing Instructions
| Size | Price / USD | Stock | Quantity |
| 5MG | 38 | Get Quote |


|
| 10MG | 52 | Get Quote |


|
| 25MG | 98 | Get Quote |


|
| 50MG | 189 | Get Quote |


|
| 100MG | 363 | Get Quote |


|
| 200MG | Get Quote | Get Quote |


|
| 500MG | Get Quote | Get Quote |


|
| 1G | Get Quote | Get Quote |


|
Biological Information
-
Product Name8-Hydroxyquinoline-2-carboxaldehyde
-
NoteResearch use only, not for human use.
-
Brief Description8-Hydroxyquinoline-2-carbaldehyde is a biochemical reagent that can be used as a biological material or organic compound for life science related research.
-
Description8-Hydroxyquinoline-2-carbaldehyde is a biochemical reagent that can be used as a biological material or organic compound for life science related research.
-
In Vitro——
-
In Vivo——
-
Synonyms——
-
PathwayOthers
-
TargetOther Targets
-
RecptorOthers
-
Research Area——
-
Indication——
Chemical Information
-
CAS Number14510-06-6
-
Formula Weight173.17
-
Molecular FormulaC10H7NO2
-
Purity>98% (HPLC)
-
Solubility——
-
SMILESOc1cccc2ccc(C=O)nc12
-
Chemical Name——
Shipping & Storage Information
-
Storage(-20℃)
-
ShippingWith Ice Pack
-
Stability≥ 2 years
Reference
molnova catalog



related products
-
Glycerite
Polyphenolic compounds with molecular weights of around 500-3000 daltons and containing enough hydroxyl groups (1-2 per 100 MW) for effective cross linking of other compounds (ASTRINGENTS).
-
Alpinumisoflavone
Alpinumisoflavone has atheroprotective effects, may result from their ability to upregulate mechanisms promoting HDL-cholesterol and bile acid formation, it is endowed with estrogenic properties accounting, at least in part, for E. lysistemon effects.
-
Metamorphosin A
Metamorphosin A



 Cart
Cart
 sales@molnova.com
sales@molnova.com


