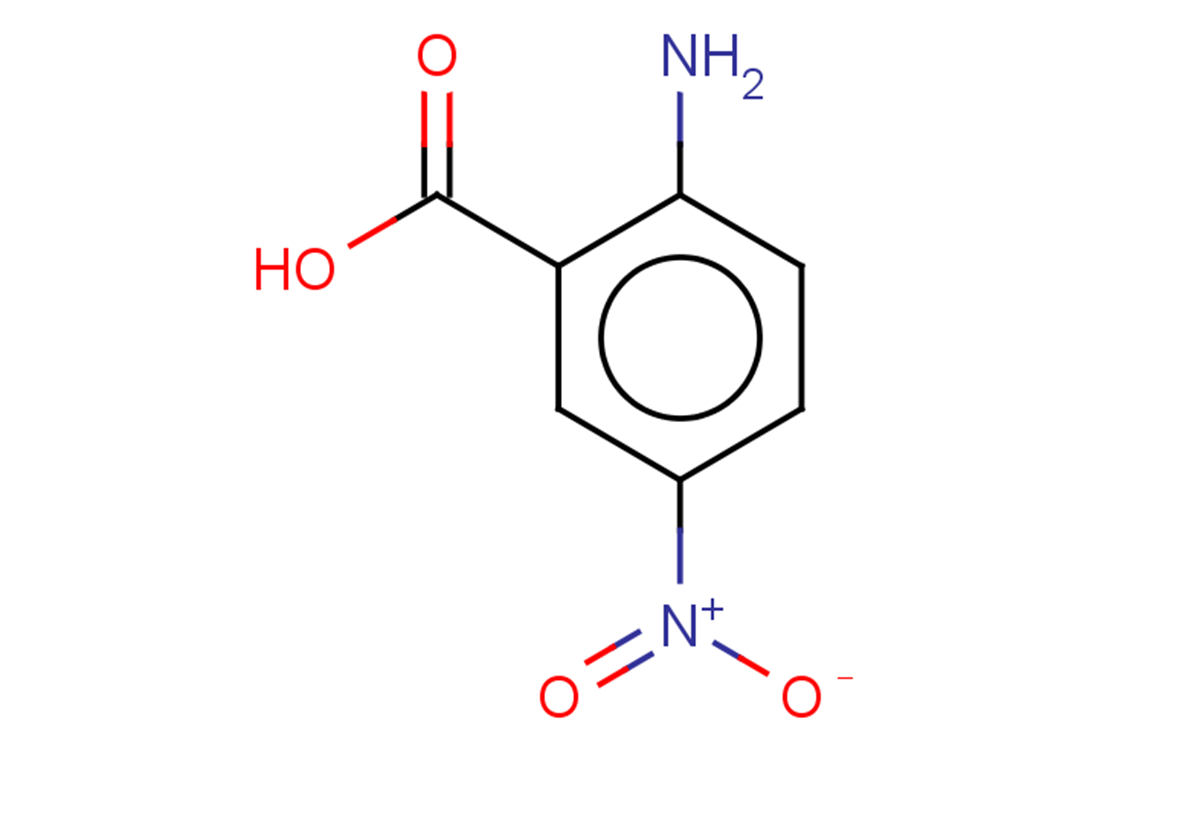
5NAA
CAS No. 616-79-5
5NAA( 5 NAA | 5-NAA )
Catalog No. M24617 CAS No. 616-79-5
5NAA is a nitroaromatic compound. 5NAA is also the starting material for synthetic dyes and other nitroaromatic compounds. 5NAA is a molecule secreted by Streptomyces scabies.
Purity : >98% (HPLC)
 COA
COA
 Datasheet
Datasheet
 HNMR
HNMR
 HPLC
HPLC
 MSDS
MSDS
 Handing Instructions
Handing Instructions
| Size | Price / USD | Stock | Quantity |
| 5MG | 45 | In Stock |


|
| 10MG | 68 | In Stock |


|
| 25MG | 115 | In Stock |


|
| 50MG | 173 | In Stock |


|
| 100MG | 258 | In Stock |


|
| 200MG | 388 | In Stock |


|
| 500MG | 642 | In Stock |


|
| 1G | Get Quote | In Stock |


|
Biological Information
-
Product Name5NAA
-
NoteResearch use only, not for human use.
-
Brief Description5NAA is a nitroaromatic compound. 5NAA is also the starting material for synthetic dyes and other nitroaromatic compounds. 5NAA is a molecule secreted by Streptomyces scabies.
-
Description5NAA is a nitroaromatic compound. 5NAA is also the starting material for synthetic dyes and other nitroaromatic compounds. 5NAA is a molecule secreted by Streptomyces scabies.
-
In Vitro——
-
In Vivo——
-
Synonyms5 NAA | 5-NAA
-
PathwayOthers
-
TargetOther Targets
-
RecptorOthers
-
Research Area——
-
Indication——
Chemical Information
-
CAS Number616-79-5
-
Formula Weight182.13
-
Molecular FormulaC7H6N2O4
-
Purity>98% (HPLC)
-
SolubilityDMSO:Soluble
-
SMILESNc(ccc([N+]([O-])=O)c1)c1C(O)=O
-
Chemical Name——
Shipping & Storage Information
-
Storage(-20℃)
-
ShippingWith Ice Pack
-
Stability≥ 2 years
Reference
1.Kalyoncu S, Heaner DP Jr, Kurt Z, Bethel CM, Ukachukwu CU, Chakravarthy S, Spain JC, Lieberman RL. Enzymatic hydrolysis by transition-metal-dependent nucleophilic aromatic substitution. Nat Chem Biol. 2016 Dec;12(12):1031-1036. doi: 10.1038/nchembio.2191.
molnova catalog



related products
-
YRGDS Fibronectin Fr...
This is a fibronectin fragment, an adhesion peptide that displays strong binding affinity to thrombin-stimulated platelets. It is RGD consisting of sequence.
-
Leucokinin V
Leucokinin V
-
MKC3946
MKC3946 is an effective and soluble IRE1α inhibitor which triggered modest growth inhibition in multiple myeloma cell lines.



 Cart
Cart
 sales@molnova.com
sales@molnova.com


