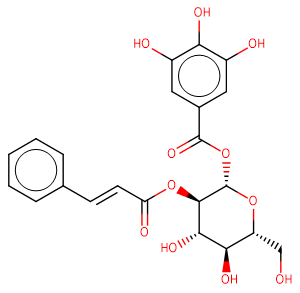
2-O-cinnamoyl-1-O-galloyl-β-D-glucose
CAS No. 791836-69-6
2-O-cinnamoyl-1-O-galloyl-β-D-glucose( —— )
Catalog No. M21707 CAS No. 791836-69-6
2-O-cinnamoyl-1-O-galloyl-β-D-glucose is a natural product.
Purity : >98% (HPLC)
 COA
COA
 Datasheet
Datasheet
 HNMR
HNMR
 HPLC
HPLC
 MSDS
MSDS
 Handing Instructions
Handing Instructions
| Size | Price / USD | Stock | Quantity |
| 5MG | 1396 | Get Quote |


|
| 100MG | Get Quote | Get Quote |


|
| 200MG | Get Quote | Get Quote |


|
| 500MG | Get Quote | Get Quote |


|
| 1G | Get Quote | Get Quote |


|
Biological Information
-
Product Name2-O-cinnamoyl-1-O-galloyl-β-D-glucose
-
NoteResearch use only, not for human use.
-
Brief Description2-O-cinnamoyl-1-O-galloyl-β-D-glucose is a natural product.
-
Description2-O-cinnamoyl-1-O-galloyl-β-D-glucose is a natural product.
-
In Vitro——
-
In Vivo——
-
Synonyms——
-
PathwayOthers
-
TargetOther Targets
-
RecptorOthers
-
Research Area——
-
Indication——
Chemical Information
-
CAS Number791836-69-6
-
Formula Weight462.4
-
Molecular FormulaC22H22O11
-
Purity>98% (HPLC)
-
Solubility——
-
SMILESO=C(O[C@@H]2O[C@H](CO)[C@@H](O)[C@H](O)[C@H]2OC(=O)/C=C/c1ccccc1)c3cc(O)c(O)c(O)c3
-
Chemical Name——
Shipping & Storage Information
-
Storage(-20℃)
-
ShippingWith Ice Pack
-
Stability≥ 2 years
Reference
molnova catalog



related products
-
SH2 Domain Ligand (1...
Ac-Asp-Tyr(PO3H2)-Val-Pro-Met-Leu-NH2 is the SH2 domain ligand. SH2 domains participate in protein tyrosine kinase (PTK)-mediated cellular signal.
-
Daphnin
Daphnin is one of the main coumarin bioactive ingredients with antibacterial activity. Daphnine is isolated from the entire Daphne fragrance, which is a folk medicine used in China to relieve fever.
-
Neuropeptide S (huma...
Potent endogenous neuropeptide S receptor agonist (EC50 = 9.4 nM). Increases locomotor activity and wakefulness in mice. Also reduces anxiety-like behavior in mice.



 Cart
Cart
 sales@molnova.com
sales@molnova.com


