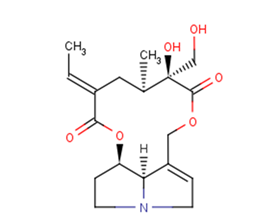
Usaramine
CAS No. 15503-87-4
Usaramine( —— )
Catalog No. M18078 CAS No. 15503-87-4
Usaramine demonstrates phytotoxicity against Lactuca sativa var. Carrascoy (lettuce) assessed as inhibition of seed germination at 50 ug/cm2 after 24 hr.
Purity : >98% (HPLC)
 COA
COA
 Datasheet
Datasheet
 HNMR
HNMR
 HPLC
HPLC
 MSDS
MSDS
 Handing Instructions
Handing Instructions
| Size | Price / USD | Stock | Quantity |
| 5MG | 248 | In Stock |


|
| 10MG | 419 | In Stock |


|
| 25MG | 689 | In Stock |


|
| 50MG | 963 | In Stock |


|
| 100MG | Get Quote | In Stock |


|
| 200MG | Get Quote | In Stock |


|
| 500MG | Get Quote | In Stock |


|
| 1G | Get Quote | In Stock |


|
Biological Information
-
Product NameUsaramine
-
NoteResearch use only, not for human use.
-
Brief DescriptionUsaramine demonstrates phytotoxicity against Lactuca sativa var. Carrascoy (lettuce) assessed as inhibition of seed germination at 50 ug/cm2 after 24 hr.
-
DescriptionUsaramine demonstrates phytotoxicity against Lactuca sativa var. Carrascoy (lettuce) assessed as inhibition of seed germination at 50 ug/cm2 after 24 hr.
-
In Vitro——
-
In Vivo——
-
Synonyms——
-
PathwayOthers
-
TargetOther Targets
-
RecptorOthers
-
Research Area——
-
Indication——
Chemical Information
-
CAS Number15503-87-4
-
Formula Weight351.39
-
Molecular FormulaC18H25NO6
-
Purity>98% (HPLC)
-
SolubilityIn Vitro:?DMSO : 100 mg/mL (284.58 mM)
-
SMILESCC=C1CC(C(C(=O)OCC2=CCN3C2C(CC3)OC1=O)(CO)O)C
-
Chemical Name——
Shipping & Storage Information
-
Storage(-20℃)
-
ShippingWith Ice Pack
-
Stability≥ 2 years
Reference
molnova catalog



related products
-
Monocrotaline N-Oxid...
Monocrotaline N-Oxide is a monocrotaline metabolite.
-
DL-Methionine Methyl...
DL-Methionine Methylsulfonium Chloride is a natural product?used in the treatment of peptic ulcers colitis and gastritis.
-
Orotirelin
Orotirelin(CG 3509), a thyrotropin-releasing hormone analog, reversed pentobarbital-induced sleep time.Orotirelin may be beneficial in animals with focal cerebral ischemia.



 Cart
Cart
 sales@molnova.com
sales@molnova.com


