Suloctidil
CAS No. 54767-75-8
Suloctidil( Suloctidil | Bemperil | Cerebro | Circleton | CP-556S | Daufan | Dulai | Hemoantin | MJF 12637 )
Catalog No. M14970 CAS No. 54767-75-8
Suloctidil is a new drug that is currently being evaluated in many clinical trials for use in dementia and thrombotic disorders.
Purity : >98% (HPLC)
 COA
COA
 Datasheet
Datasheet
 HNMR
HNMR
 HPLC
HPLC
 MSDS
MSDS
 Handing Instructions
Handing Instructions
| Size | Price / USD | Stock | Quantity |
| 100MG | 229 | In Stock |


|
| 200MG | 385 | In Stock |


|
| 500MG | Get Quote | In Stock |


|
| 1G | Get Quote | In Stock |


|
Biological Information
-
Product NameSuloctidil
-
NoteResearch use only, not for human use.
-
Brief DescriptionSuloctidil is a new drug that is currently being evaluated in many clinical trials for use in dementia and thrombotic disorders.
-
DescriptionSuloctidil is a new drug that is currently being evaluated in many clinical trials for use in dementia and thrombotic disorders.
-
In Vitro——
-
In Vivo——
-
SynonymsSuloctidil | Bemperil | Cerebro | Circleton | CP-556S | Daufan | Dulai | Hemoantin | MJF 12637
-
PathwayOthers
-
TargetPlatelet aggregation
-
RecptorAntiplatelet-aggregation
-
Research AreaCardiovascular Disease
-
Indication——
Chemical Information
-
CAS Number54767-75-8
-
Formula Weight337.56
-
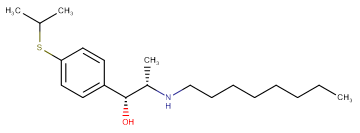
Molecular FormulaC20H35NOS
-
Purity>98% (HPLC)
-
SolubilityDMSO: 10 mM
-
SMILESO[C@@H]([C@@H](NCCCCCCCC)C)C1=CC=C(SC(C)C)C=C1
-
Chemical Name(1R,2S)-1-(4-(isopropylthio)phenyl)-2-(octylamino)propan-1-ol
Shipping & Storage Information
-
Storage(-20℃)
-
ShippingWith Ice Pack
-
Stability≥ 2 years
Reference
1. Ishibashi A, et al. Nihon Yakurigaku Zasshi. 1983 Nov;82(5):361-73.
molnova catalog



related products
-
ethyl 1-[(4-cyanoben...
ethyl 1-[(4-cyanobenzyl)oxy]-2-(3-cyanophenyl)-4-methyl-1H-imidazole-5-carboxylate is a platelet aggregation inhibitor.
-
Mutant EGFR inhibito...
Mutant EGFR inhibitor is a selective and potent Mutated EGFR inhibitor, inhibits L858R activating mutant, the Exonl9 deletion activating mutant and the T790M resistance mutant.
-
Etizolam
Etizolam is a selective platelet-activating factor (PAF) antagonist.



 Cart
Cart
 sales@molnova.com
sales@molnova.com


