PF-04457845
CAS No. 1020315-31-4
PF-04457845( —— )
Catalog No. M17118 CAS No. 1020315-31-4
PF-04457845 is a greatly and effctive FAAH inhibitor, and for hFAAH(IC50=7.2±0.63 nM) and rFAAH(IC50=7.4±0.62 nM).
Purity : >98% (HPLC)
 COA
COA
 Datasheet
Datasheet
 HNMR
HNMR
 HPLC
HPLC
 MSDS
MSDS
 Handing Instructions
Handing Instructions
| Size | Price / USD | Stock | Quantity |
| 2MG | 27 | In Stock |


|
| 5MG | 43 | In Stock |


|
| 10MG | 73 | In Stock |


|
| 25MG | 149 | In Stock |


|
| 50MG | 222 | In Stock |


|
| 100MG | 397 | In Stock |


|
| 200MG | 516 | In Stock |


|
| 500MG | Get Quote | In Stock |


|
| 1G | Get Quote | In Stock |


|
Biological Information
-
Product NamePF-04457845
-
NoteResearch use only, not for human use.
-
Brief DescriptionPF-04457845 is a greatly and effctive FAAH inhibitor, and for hFAAH(IC50=7.2±0.63 nM) and rFAAH(IC50=7.4±0.62 nM).
-
DescriptionPF-04457845 is a greatly and effctive FAAH inhibitor, and for hFAAH(IC50=7.2±0.63 nM) and rFAAH(IC50=7.4±0.62 nM).(In Vitro):PF-04457845 inhibits FAAH by a covalent, irreversible mechanism involving carbamylation of the active-site serine nucleophile of FAAH with high in vitro potency (kinact/Ki and IC50 values of 40300 M-1s-1 and 7.2 nM, respectively, for human FAAH). PF-04457845 has exquisite selectivity for FAAH relative to other members of the serine hydrolase superfamily as demonstrated by competitive activity-based protein profiling. PF-04457845 completely inhibits FAAH in human and mouse membrane proteomes at both 10 and 100 μM with no off targets. PF-04457845 is completely selective for FAAH, and none of the other FP-reactive serine hydrolases in the tested tissues are inhibited by PF-04457845 even at 100 μM. (In Vivo):Oral administration of PF-04457845 at 0.1 mg/kg results in efficacy comparable to that of naproxen at 10 mg/kg in a rat model of inflammatory pain. Oral administration of PF-04457845 causes a significant inhibition of mechanical allodynia measured after 4 h with a minimum effective dose (MED) of 0.1 mg/kg. Furthermore, at 0.1 mg/kg (p.o.), PF-04457845 inhibits the pain response to a comparable degree as the nonsteroidal anti-inflammatory drug naproxen at 10 mg/kg. FAAH is confirmed to be completely inhibited in mice treated with PF-04457845 at 1 and 10 mg/kg p.o. by competitive activity-based protein profiling (ABPP) study.
-
In VitroRedafamdastat inhibits FAAH by a covalent, irreversible mechanism involving carbamylation of the active-site serine nucleophile of FAAH with high in vitro potency (kinact/Ki and IC50 values of 40300 M-1s-1 and 7.2 nM, respectively, for human FAAH). Redafamdastat has exquisite selectivity for FAAH relative to other members of the serine hydrolase superfamily as demonstrated by competitive activity-based protein profiling. Redafamdastat completely inhibits FAAH in human and mouse membrane proteomes at both 10 and 100 μM with no off targets. Redafamdastat is completely selective for FAAH, and none of the other FP-reactive serine hydrolases in the tested tissues are inhibited by Redafamdastat even at 100 μM.
-
In VivoOral administration of Redafamdastat at 0.1 mg/kg results in efficacy comparable to that of naproxen at 10 mg/kg in a rat model of inflammatory pain. Oral administration of Redafamdastat causes a significant inhibition of mechanical allodynia measured after 4 h with a minimum effective dose (MED) of 0.1 mg/kg. Furthermore, at 0.1 mg/kg (p.o.), Redafamdastat inhibits the pain response to a comparable degree as the nonsteroidal anti-inflammatory drug naproxen at 10 mg/kg. FAAH is confirmed to be completely inhibited in mice treated with Redafamdastat at 1 and 10 mg/kg p.o. by competitive activity-based protein profiling (ABPP) study.
-
Synonyms——
-
PathwayOthers
-
TargetOther Targets
-
RecptorhFAAH|rFAAH
-
Research AreaInflammation/Immunology
-
Indication——
Chemical Information
-
CAS Number1020315-31-4
-
Formula Weight455.44
-
Molecular FormulaC23H20F3N5O2
-
Purity>98% (HPLC)
-
SolubilityDMSO : ≥ 100 mg/mL 219.57 mM
-
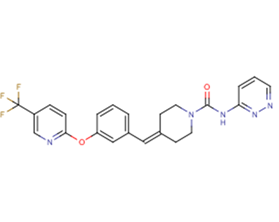
SMILESC1CN(CCC1=CC2=CC(=CC=C2)OC3=NC=C(C=C3)C(F)(F)F)C(=O)NC4=NN=CC=C4
-
Chemical Name——
Shipping & Storage Information
-
Storage(-20℃)
-
ShippingWith Ice Pack
-
Stability≥ 2 years
Reference
1.Johnson DS, et al. ACS Med Chem Lett. 2011 Feb 10;2(2):91-96.
molnova catalog



related products
-
4-Methoxybenzoylacet...
The roots of Asarum sieboldii Miq.
-
Parishin E
Parishin E is a natural product from Gastrodia elata,have antioxidant property.
-
Mefexamide hydrochlo...
Mefexamide hydrochloride is a psychostimulant.



 Cart
Cart
 sales@molnova.com
sales@molnova.com


