Mifepristone
CAS No. 84371-65-3
Mifepristone( RU-486 )
Catalog No. M16134 CAS No. 84371-65-3
Mifepristone is a progestational and glucocorticoid hormone antagonist.
Purity : >98% (HPLC)
 COA
COA
 Datasheet
Datasheet
 HNMR
HNMR
 HPLC
HPLC
 MSDS
MSDS
 Handing Instructions
Handing Instructions
| Size | Price / USD | Stock | Quantity |
| 50MG | 33 | In Stock |


|
| 100MG | 43 | In Stock |


|
| 200MG | 55 | In Stock |


|
| 500MG | 76 | In Stock |


|
| 1G | Get Quote | In Stock |


|
Biological Information
-
Product NameMifepristone
-
NoteResearch use only, not for human use.
-
Brief DescriptionMifepristone is a progestational and glucocorticoid hormone antagonist.
-
DescriptionMifepristone is a progestational and glucocorticoid hormone antagonist. Its inhibition of progesterone induces bleeding during the luteal phase and in early pregnancy by releasing endogenous prostaglandins from the endometrium or decidua. As a glucocorticoid receptor antagonist, the drug has been used to treat hypercortisolism in patients with nonpituitary cushing syndrome.(In Vitro):The discovery of the first competitive progesterone antagonist, Mifepristone, has stimulated an intense search for more potent and more selective antiprogestins. Cell growth is evaluated after 4 days of exposure to Mifepristone at 10 μM, a concentration close to the plasma concentration achievable in humans. The antiproliferative effect of NSC 119875 is potentiated when administered in combination with Mifepristone in HeLa cells. The IC50 of NSC 119875 in combination with Mifepristone is lower (14.2 μM) than that of NSC 119875 alone (34.2 μM) in HeLa cells with an approximately 2.5-fold difference. After treatment with Mifepristone, the accumulation of intracellular NSC 119875 in HeLa cells is 2-fold greater, representing a significant difference (p=0.009), compare with NSC 119875 alone from 0.79 to 1.52 μg/mg of protein. (In Vivo):The cervix tumor xenograft models are treated with NSC 119875 alone, there is a tumor growth inhibition compare with control group. However, the tumor weight loss is even more significant (p<0.05) with the combination of NSC 119875 and Mifepristone at the doses used, showing a decrease of ~50% compared with the treatments alone by the end of the study. Adult male Sprague-Dawley rats are subjected to a 4-day binge-like EtOH administration regimen (3 to 5 g/kg/i.g. every 8 hours designed to produce peak blood EtOH levels (BELs) of <300 mg/dL). Subgroups of animals receive s.c. injection of Mifepristone (20 or 40 mg/kg in peanut oil). Although Mifepristone produces no significant changes in behavior of EtOH-na ve animals, pretreatment with Mifepristone (40 mg/kg) significantly reducesthe severity of EtOH withdrawal. Asignificant interaction between diet and drug, F(5,55)=3.92, p<0.05, such that EtOH-treated animals receiving vehicle or 20 mg/kg of Mifepristone displayssignificantly more signs of EtOH withdrawal than does EtOH-na ve animals receiving the same drug treatment. Importantly, treatment with 40 mg/kg of Mifepristone significantly reduces the severity of EtOH withdrawal, in a dose-dependent manner.
-
In VitroThe discovery of the first competitive progesterone antagonist, Mifepristone, has stimulated an intense search for more potent and more selective antiprogestins. Cell growth is evaluated after 4 days of exposure to Mifepristone at 10 μM, a concentration close to the plasma concentration achievable in humans. The antiproliferative effect of NSC 119875 is potentiated when administered in combination with Mifepristone in HeLa cells. The IC50 of NSC 119875 in combination with Mifepristone is lower (14.2 μM) than that of NSC 119875 alone (34.2 μM) in HeLa cells with an approximately 2.5-fold difference. After treatment with Mifepristone, the accumulation of intracellular NSC 119875 in HeLa cells is 2-fold greater, representing a significant difference (p=0.009), compare with NSC 119875 alone from 0.79 to 1.52 μg/mg of protein.
-
In VivoThe cervix tumor xenograft models are treated with NSC 119875 alone, there is a tumor growth inhibition compare with control group. However, the tumor weight loss is even more significant (p<0.05) with the combination of NSC 119875 and Mifepristone at the doses used, showing a decrease of ~50% compared with the treatments alone by the end of the study. Adult male Sprague-Dawley rats are subjected to a 4-day binge-like EtOH administration regimen (3 to 5 g/kg/i.g. every 8 hours designed to produce peak blood EtOH levels (BELs) of <300 mg/dL). Subgroups of animals receive s.c. injection of Mifepristone (20 or 40 mg/kg in peanut oil). Although Mifepristone produces no significant changes in behavior of EtOH-na?ve animals, pretreatment with Mifepristone (40 mg/kg) significantly reducesthe severity of EtOH withdrawal. Asignificant interaction between diet and drug, F(5,55)=3.92, p<0.05, such that EtOH-treated animals receiving vehicle or 20 mg/kg of Mifepristone displayssignificantly more signs of EtOH withdrawal than does EtOH-na?ve animals receiving the same drug treatment. Importantly, treatment with 40 mg/kg of Mifepristone significantly reduces the severity of EtOH withdrawal, in a dose-dependent manner.
-
SynonymsRU-486
-
PathwayEndocrinology/Hormones
-
TargetEstrogen Receptor/ERR
-
RecptorPR| Glucocorticoid Receptor
-
Research AreaEndocrinology
-
Indication——
Chemical Information
-
CAS Number84371-65-3
-
Formula Weight429.6
-
Molecular FormulaC29H35NO2
-
Purity>98% (HPLC)
-
SolubilityEthanol: 19 mg/mL (44.22 mM); DMSO: 85 mg/mL (197.86 mM)
-
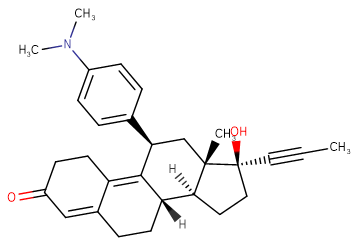
SMILESO=C1CCC2=C3[C@@H](C4=CC=C(N(C)C)C=C4)C[C@]5(C)[C@@](C#CC)(O)CC[C@@]5([H])[C@]3([H])CCC2=C1
-
Chemical Name(8S,11R,13S,14S,17S)-11-(4-(dimethylamino)phenyl)-17-hydroxy-13-methyl-17-(prop-1-yn-1-yl)-6,7,8,11,12,13,14,15,16,17-decahydro-1H-cyclopenta[a]phenanthren-3(2H)-one
Shipping & Storage Information
-
Storage(-20℃)
-
ShippingWith Ice Pack
-
Stability≥ 2 years
Reference
1.Jiang W, et al. Bioorg Med Chem, 2006, 14(19), 6726-6732.
molnova catalog



related products
-
Progesterone
Progesterone is a critical hormone for the maintenance of pregnancy, menstrual cycle, endometrium and functions with its receptor
-
Ethynyl estradiol
Ethynyl estradiol is an orally bio-active estrogen used in almost all modern formulations of combined oral contraceptive pills.
-
WAY-200070
WAY-200070 is a potent, highly selective ERβ agonist with IC50 of 3 nM, displays 68-fold selectivity over ERα (IC50=260 nM).



 Cart
Cart
 sales@molnova.com
sales@molnova.com


