ML265
CAS No. 1221186-53-3
ML265( TEPP-46 )
Catalog No. M20635 CAS No. 1221186-53-3
ML265 is a potent PKM2 activator (AC50 : 92 nM) showing little or no effect on PKM1 PKL and PKRinduces tetramerization and reduces tumor formation and size in a mouse xenograft model.?.
Purity : >98% (HPLC)
 COA
COA
 Datasheet
Datasheet
 HNMR
HNMR
 HPLC
HPLC
 MSDS
MSDS
 Handing Instructions
Handing Instructions
| Size | Price / USD | Stock | Quantity |
| 5MG | 87 | In Stock |


|
| 10MG | 132 | In Stock |


|
| 50MG | 336 | In Stock |


|
| 100MG | Get Quote | In Stock |


|
| 200MG | Get Quote | In Stock |


|
| 500MG | Get Quote | In Stock |


|
| 1G | Get Quote | In Stock |


|
Biological Information
-
Product NameML265
-
NoteResearch use only, not for human use.
-
Brief DescriptionML265 is a potent PKM2 activator (AC50 : 92 nM) showing little or no effect on PKM1 PKL and PKRinduces tetramerization and reduces tumor formation and size in a mouse xenograft model.?.
-
DescriptionML265 is a potent PKM2 activator (AC50 : 92 nM) showing little or no effect on PKM1 PKL and PKRinduces tetramerization and reduces tumor formation and size in a mouse xenograft model.?(In Vitro):TEPP-46 and DASA-58 activate PKM2 by a mechanism similar to that of the endogenous activator FBP. Pre-treatment of cells with TEPP-46 or DASA-58 prevents pervanadate-induced inhibition of PKM2 activity. TEPP-46 also induces a decrease in the intracellular levels of acetyl-coA, lactate, ribose phosphate and serine. TEPP-46 inhibits LPS-induced Hif-1α and IL-1β, as well as the expression of a range of other Hif-1α-dependent genes. TEPP-46 treatment significantly downregulates the expression of the M1 markers Il12p40 and Cxcl-10. Activation of PKM2 using TEPP-46 significantly inhibits FSL-1 and CpG-induced Il1b mRNA expression. TEPP-46 inhibits Mtb-induced Il1b mRNA levels, boosts Mtb-induced levels of Il10 mRNA, and has no effect on levels of Tnf.(In Vivo):TEPP-46 exhibits good oral bioavailability with relatively low clearance, long half-life, and good volume of distribution-parameters that predict for drug exposure in tumor tissues. TEPP-46 at 150 mg/kg readily achieves maximal PKM2 activation measured in A549 xenograft tumors.
-
In VitroTEPP-46 and DASA-58 activate PKM2 by a mechanism similar to that of the endogenous activator FBP. Pre-treatment of cells with TEPP-46 or DASA-58 prevents pervanadate-induced inhibition of PKM2 activity. TEPP-46 also induces a decrease in the intracellular levels of acetyl-coA, lactate, ribose phosphate and serine. TEPP-46 inhibits LPS-induced Hif-1α and IL-1β, as well as the expression of a range of other Hif-1α-dependent genes. TEPP-46 treatment significantly downregulates the expression of the M1 markers Il12p40 and Cxcl-10. Activation of PKM2 using TEPP-46 significantly inhibits FSL-1 and CpG-induced Il1b mRNA expression. TEPP-46 inhibits Mtb-induced Il1b mRNA levels, boosts Mtb-induced levels of Il10 mRNA, and has no effect on levels of Tnf.
-
In VivoTEPP-46 exhibits good oral bioavailability with relatively low clearance, long half-life, and good volume of distribution-parameters that predict for drug exposure in tumor tissues. TEPP-46 at 150 mg/kg readily achieves maximal PKM2 activation measured in A549 xenograft tumors.
-
SynonymsTEPP-46
-
PathwayOthers
-
TargetOther Targets
-
RecptorPKM2
-
Research Area——
-
Indication——
Chemical Information
-
CAS Number1221186-53-3
-
Formula Weight372.46
-
Molecular FormulaC17H16N4O2S2
-
Purity>98% (HPLC)
-
SolubilityDMSO:50 mg/mL (134.24 mM)
-
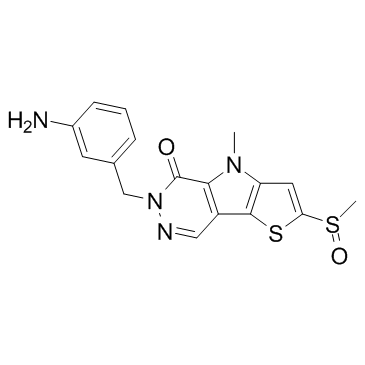
SMILESCn1c2cc(sc2c2cnn(Cc3cccc(N)c3)c(=O)c12)S(C)=O
-
Chemical Name6-(3-aminobenzyl)-4-methyl-2-(methylsulfinyl)-46-dihydro-5H-thieno[2'3':45]pyrrolo[23-d]pyridazin-5-one
Shipping & Storage Information
-
Storage(-20℃)
-
ShippingWith Ice Pack
-
Stability≥ 2 years
Reference
1.Walsh M J Brimacombe K R Anastasiou D et al. ML265: A potent PKM2 activator induces tetramerization and reduces tumor formation and size in a mouse xenograft model[M]// Probe Reports from the NIH Molecular Libraries Program. PubMed 2009.
molnova catalog



related products
-
MS-PEG4-THP
MS-PEG4-THP is a PROTAC linker, which is a PEG type.
-
1,7-Bis(4-hydroxyphe...
(4Z,6E)-5-hydroxy-1,7-bis(4-hydroxyphenyl)hepta-4,6-dien-3-one shows growth inhibitory activity in vitro versus bloodstream forms of African trypanosomes, with the IC(50) value in the range of 1-3 microg/mL.
-
Oseltamivir Impurity...
3-Des(1-ethylpropoxy)-3-(1-methylpropoxy) Oseltamivir (Oseltamivir EP Impurity F) is an impurity of the antiviral drug Oseltamivir.



 Cart
Cart
 sales@molnova.com
sales@molnova.com


