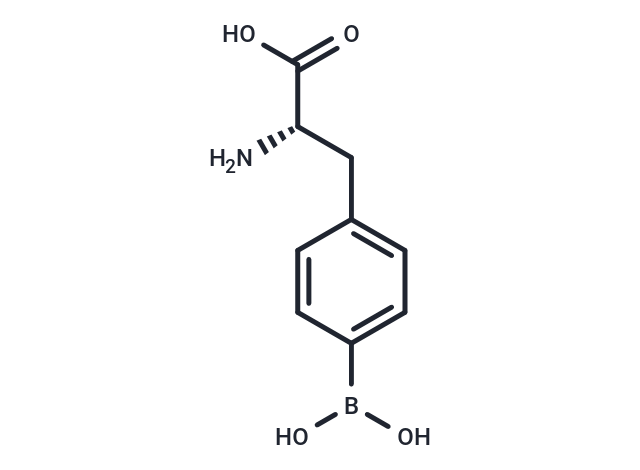
4-Borono-L-phenylalanine
CAS No. 76410-58-7
4-Borono-L-phenylalanine( —— )
Catalog No. M37069 CAS No. 76410-58-7
4-Borono-L-phenylalanine has antitumor activity and may be used in clinical trials of boron neutron capture therapy for the treatment of melanoma and glioblastoma multiforme.
Purity : >98% (HPLC)
 COA
COA
 Datasheet
Datasheet
 HNMR
HNMR
 HPLC
HPLC
 MSDS
MSDS
 Handing Instructions
Handing Instructions
| Size | Price / USD | Stock | Quantity |
| 100MG | 27 | In Stock |


|
| 200MG | Get Quote | In Stock |


|
| 500MG | Get Quote | In Stock |


|
| 1G | Get Quote | In Stock |


|
Biological Information
-
Product Name4-Borono-L-phenylalanine
-
NoteResearch use only, not for human use.
-
Brief Description4-Borono-L-phenylalanine has antitumor activity and may be used in clinical trials of boron neutron capture therapy for the treatment of melanoma and glioblastoma multiforme.
-
Description(S)-2-Amino-3-(4-boronophenyl)propanoic acid is a biochemical reagent that can be used as a biological material or organic compound for life science related research.
-
In Vitro——
-
In Vivo——
-
Synonyms——
-
PathwayOthers
-
TargetOther Targets
-
RecptorOthers
-
Research Area——
-
Indication——
Chemical Information
-
CAS Number76410-58-7
-
Formula Weight209.01
-
Molecular FormulaC9H12BNO4
-
Purity>98% (HPLC)
-
Solubility——
-
SMILESN[C@@H](Cc1ccc(cc1)B(O)O)C(O)=O
-
Chemical Name——
Shipping & Storage Information
-
Storage(-20℃)
-
ShippingWith Ice Pack
-
Stability≥ 2 years
Reference
molnova catalog



related products
-
TRAP-6 amide
TRAP-6 amide is a PAR-1 thrombin receptor agonist peptide.
-
Fezakinumab
Fezakinumab is a potent monoclonal antibody to interleukin-22 (IL-22). Fezakinumab can be used to study immune system diseases and inflammation-related conditions such as psoriasis, rheumatoid arthritis and atopic dermatitis.
-
Eupteleasaponin I
Eupteleasaponin I is a natural product from Euptelea polyandra Sieb. et Zucc.



 Cart
Cart
 sales@molnova.com
sales@molnova.com


