Flibanserin
CAS No. 167933-07-5
Flibanserin( EBD6396 | Flibanserin. Brand name: Addyi )
Catalog No. M18110 CAS No. 167933-07-5
Flibanserin is a serotonergic antidepressant used to treat hypoactive sexual desire disorder.
Purity : >98% (HPLC)
 COA
COA
 Datasheet
Datasheet
 HNMR
HNMR
 HPLC
HPLC
 MSDS
MSDS
 Handing Instructions
Handing Instructions
| Size | Price / USD | Stock | Quantity |
| 1 mL x 10 mM in DMSO | 37 | In Stock |


|
| 5MG | 32 | In Stock |


|
| 10MG | 56 | In Stock |


|
| 25MG | 104 | In Stock |


|
| 50MG | 183 | In Stock |


|
| 100MG | 237 | In Stock |


|
| 200MG | 352 | In Stock |


|
| 500MG | Get Quote | In Stock |


|
| 1G | Get Quote | In Stock |


|
Biological Information
-
Product NameFlibanserin
-
NoteResearch use only, not for human use.
-
Brief DescriptionFlibanserin is a serotonergic antidepressant used to treat hypoactive sexual desire disorder.
-
DescriptionFlibanserin is a full agonist of the 5-HT1A receptor (Ki = 1 nM) and, with lower affinity, as an antagonist of the 5-HT2A receptor (Ki = 49 nM) and antagonist or very weak partial agonist of the D4 receptor (Ki = 4–24 nM). Despite the much greater affinity of flibanserin for the 5-HT1A receptor, and for reasons that are unknown, flibanserin occupies the 5-HT1A and 5-HT2A receptors in vivo with similar percentages. Flibanserin also has low affinity for the 5-HT2B receptor (Ki = 89.3 nM) and the 5-HT2C receptor (Ki = 88.3 nM), both of which it behaves as an antagonist of. Flibanserin preferentially activates 5-HT1A receptors in the prefrontal cortex, demonstrating regional selectivity, and has been found to increase dopamine and norepinephrine levels and decrease serotonin levels in the rat prefrontal cortex, actions that were determined to be mediated by activation of the 5-HT1A receptor.[12] As such, flibanserin has been described as a norepinephrine-dopamine disinhibitor (NDDI). Flibanserin was approved in August 2015 for the treatment of pre-menopausal women with hypoactive sexual desire disorder (HSDD).(In Vitro):Flibanserin (0.01-100 μM; 72 h) can transform into two degradation products DP1 and DP2 with no toxicity potential after oxidative degradation.(In Vivo):Flibanserin (1, 10, 30 mg/kg; i.p.; single dose) shows different pharmacological properties in prefrontal cortex, hippocampus and midbrain. The 5-HT1A receptor occupancy in cortex indicates it’s the more sensitive than other brain region.Flibanserin (15, 45 mg/kg; p.o.; twice a day; 22 d) preferentially activates the brain regions belonging to the mesolimbic dopaminergic pathway and hypothalamic structures involved in the integration of sexual cues related to sexual motivation.Flibanserin (5, 10, 25, and 50 mg/kg; s.c.; single dose) has anxiolytic effects without locomotor side effects in rat ultrasonic vocalization model.
-
In VitroFlibanserin (0.01-100 μM; 72 h) can transform into two degradation products DP1 and DP2 with no toxicity potential after oxidative degradation.Cell Viability Assay Cell Line:NHSF cell lin Concentration:0.01, 0.1, 1, 10, 100 μM Incubation Time:72 hours Result:Resulted cell viability reached to 97.91% (DP1) and 96.73% (DP2) at 0.01 μM.
-
In VivoFlibanserin (1, 10, 30 mg/kg; i.p.; single dose) shows different pharmacological properties in prefrontal cortex, hippocampus and midbrain. The 5-HT1A receptor occupancy in cortex indicates it’s the more sensitive than other brain region.Flibanserin (15, 45 mg/kg; p.o.; twice a day; 22 d) preferentially activates the brain regions belonging to the mesolimbic dopaminergic pathway and hypothalamic structures involved in the integration of sexual cues related to sexual motivation.Flibanserin (5, 10, 25, and 50 mg/kg; s.c.; single dose) has anxiolytic effects without locomotor side effects in rat ultrasonic vocalization model. Animal Model:Long Evans female rats (225-250 g)Dosage:15 mg/kg; 45 mg/kg Administration:Oral gavage; twice a day for 22 daysResult:Increased the density of activated catecholaminergic neurons in the ventral tegmental area but not in the locus coeruleus.Increased Fos expression in the medial preoptic area and arcuate nucleus of the hypothalamus, ventral tegmental area, locus coeruleus, and lateral paragigantocellular nucleus with chronic 22-day treatment.Animal Model:Rat pup ultrasonic vocalization model of anxietyDosage:5, 10, 25, and 50 mg/kg Administration:Subcutaneous injection Result:Reduced ultrasonic vocalizations in rat pups.Showed effective within 30 min and has no severe locomotor side effects at active doses.
-
SynonymsEBD6396 | Flibanserin. Brand name: Addyi
-
PathwayOthers
-
TargetOther Targets
-
Recptor5-HT1A|5-HT2A
-
Research AreaNeurological Disease
-
Indication——
Chemical Information
-
CAS Number167933-07-5
-
Formula Weight390.41
-
Molecular FormulaC20H21F3N4O
-
Purity>98% (HPLC)
-
SolubilityDMSO : ≥ 45 mg/mL; 115.27 mM
-
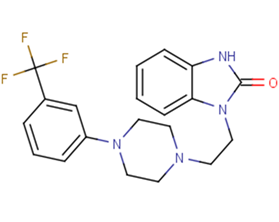
SMILESFC(F)(F)c1cccc(c1)N1CCN(CCn2c3ccccc3[nH]c2=O)CC1
-
Chemical Name1-(2-{4-[3-(Trifluoromethyl)phenyl]piperazin-1-yl}ethyl)-1,3-dihydro-2H-benzimidazol-2-one
Shipping & Storage Information
-
Storage(-20℃)
-
ShippingWith Ice Pack
-
Stability≥ 2 years
Reference
molnova catalog



related products
-
CHD1Li 6.11
CHD1Li 6.11 is a potent inhibitor of oncogenic CHD1L, acting on the cat-CHD1L recombinant protein (IC50: 3.3 μM).
-
Octisalate
Octyl salicylate, or 2-ethylhexyl salicylate, is an organic compound used as an ingredient in sunscreens and cosmetics to absorb UVB (ultraviolet) rays from the sun.
-
Cemiplimab
Cemiplimab (Anti-Human PD-1) is a human monoclonal antibody that inhibits the PD-1/PD-L1 pathway, serving as a checkpoint inhibitor.



 Cart
Cart
 sales@molnova.com
sales@molnova.com


