Veliparib
CAS No. 912444-00-9
Veliparib( ABT-888 | ABT 888 | ABT888 )
Catalog No. M16548 CAS No. 912444-00-9
Veliparib (ABT-888) is a potent, BBB penetrant, and orally active PARP inhibitor with IC50 of 5.2 and 2.9 nM for PARP-1 and PARP-2, respectively.
Purity : >98% (HPLC)
 COA
COA
 Datasheet
Datasheet
 HNMR
HNMR
 HPLC
HPLC
 MSDS
MSDS
 Handing Instructions
Handing Instructions
| Size | Price / USD | Stock | Quantity |
| 1 mL x 10 mM in DMSO | 55 | In Stock |


|
| 5MG | 49 | In Stock |


|
| 10MG | 70 | In Stock |


|
| 25MG | 123 | In Stock |


|
| 50MG | 202 | In Stock |


|
| 100MG | 325 | In Stock |


|
| 200MG | 528 | In Stock |


|
| 500MG | 834 | In Stock |


|
| 1G | Get Quote | In Stock |


|
Biological Information
-
Product NameVeliparib
-
NoteResearch use only, not for human use.
-
Brief DescriptionVeliparib (ABT-888) is a potent, BBB penetrant, and orally active PARP inhibitor with IC50 of 5.2 and 2.9 nM for PARP-1 and PARP-2, respectively.
-
DescriptionVeliparib (ABT-888) is a potent, BBB penetrant, and orally active PARP inhibitor with IC50 of 5.2 and 2.9 nM for PARP-1 and PARP-2, respectively; displays no activity against SIRT2 and selective biochemical profile in a panel of 74 receptor-binding assays; strongly potentiates temozolomide in the B16F10 s.c. murine melanoma model, also potentiates temozolomide, platinums, cyclophosphamide, and radiation in syngeneic and xenograft tumor models. Breast Cancer Phase 3 Clinical(In Vitro):Veliparib (ABT-888) is also tested against SIRT2, an enzyme that also uses NAD+ for catalysis, and found to be inactive (>5,000 nM). The receptor profile of Veliparib is determined in a panel of 74 receptor-binding assays at a concentration of 10 μM. Veliparib displaces control-specific binding at 50% or greater at the human H1(61%), the human 5-HT1A (91%), and the human 5-HT7 (84%) sites only. The IC50s for these three receptors are 5.3, 1.5, and 1.2 μM, respectively. c-Met knockdown cells show 4.2- (shMet-A; 95% CI=4-4.5) or 4.6-fold (shMet-B; 95% CI=4.4-4.8) growth inhibition when treated with 60 μM Veliparib (ABT-888). When treated with 38 μM Veliparib, c-Met knockdown cells show 2- (shMet-A; 95% CI=1.5-2.5) or 1.9-fold (shMet-B; 95% CI=1.3-2.5) growth inhibition. In HaCaT cells, at 6 h post-treatment by Veliparib (ABT-888), cell viability is significantly increases under 1,000 μM sulfur mustard (SM) exposure, whereas Veliparib does not protect cell viability under 100 μM SM exposure. Moreover, the addition of Veliparib no longer shows the protective effect at 24 h post SM exposure.(In Vivo):Veliparib (ABT-888) is a potent inhibitor of PARP, has good oral bioavailability, can cross the blood-brain barrier in syngeneic and xenograft tumor models. In MDA-MB-231 xenograft tumor models, combination treatment (AG014699/PF-02341066 and Veliparib (ABT-888)/Foretinib) substantially reduced tumor growth compared to either inhibitor alone.
-
In Vitro——
-
In Vivo——
-
SynonymsABT-888 | ABT 888 | ABT888
-
PathwayCell Cycle/DNA Damage
-
TargetPARP
-
RecptorPARP1|PARP2
-
Research AreaCancer
-
IndicationBreast Cancer
Chemical Information
-
CAS Number912444-00-9
-
Formula Weight244.2923
-
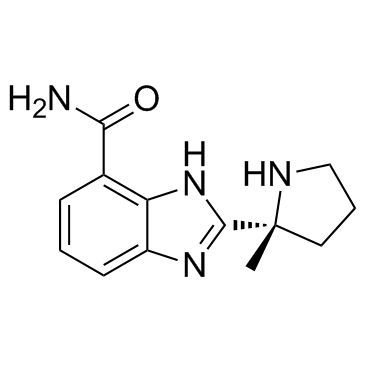
Molecular FormulaC13H16N4O
-
Purity>98% (HPLC)
-
SolubilityDMSO: ≥ 29 mg/mL
-
SMILESC[C@@]1(CCCN1)C2=NC3=C(C=CC=C3N2)C(=O)N
-
Chemical Name1H-Benzimidazole-7-carboxamide, 2-[(2R)-2-methyl-2-pyrrolidinyl]-
Shipping & Storage Information
-
Storage(-20℃)
-
ShippingWith Ice Pack
-
Stability≥ 2 years
Reference
1. Donawho CK, et al. Clin Cancer Res. 2007 May 1;13(9):2728-37.
2. Albert JM, et al. Clin Cancer Res. 2007 May 15;13(10):3033-42.
3. Liu X, et al. Mol Cancer Res. 2008 Oct;6(10):1621-9.
4. Palma JP, et al. Anticancer Res. 2008 Sep-Oct;28(5A):2625-35.
molnova catalog



related products
-
RBN-3143
RBN-3143 is a potent inhibitor of NAD+-competitive catalytic PARP14 (IC50= 4 nM), which inhibits ADP-ribosylation mediated by PARP14 and stabilizes PARP14 in cell lines, demonstrating research potential for lung inflammation.
-
WAY-620473
WAY-620473 is a potent PARP-1 inhibitor with antitumor activity and alters eukaryotic lifespan.
-
RBN012759
RBN012759, a potent and selective PARP14 inhibitor, decreases pro-tumor macrophage function and elicits inflammatory responses in tumor explants.



 Cart
Cart
 sales@molnova.com
sales@molnova.com


