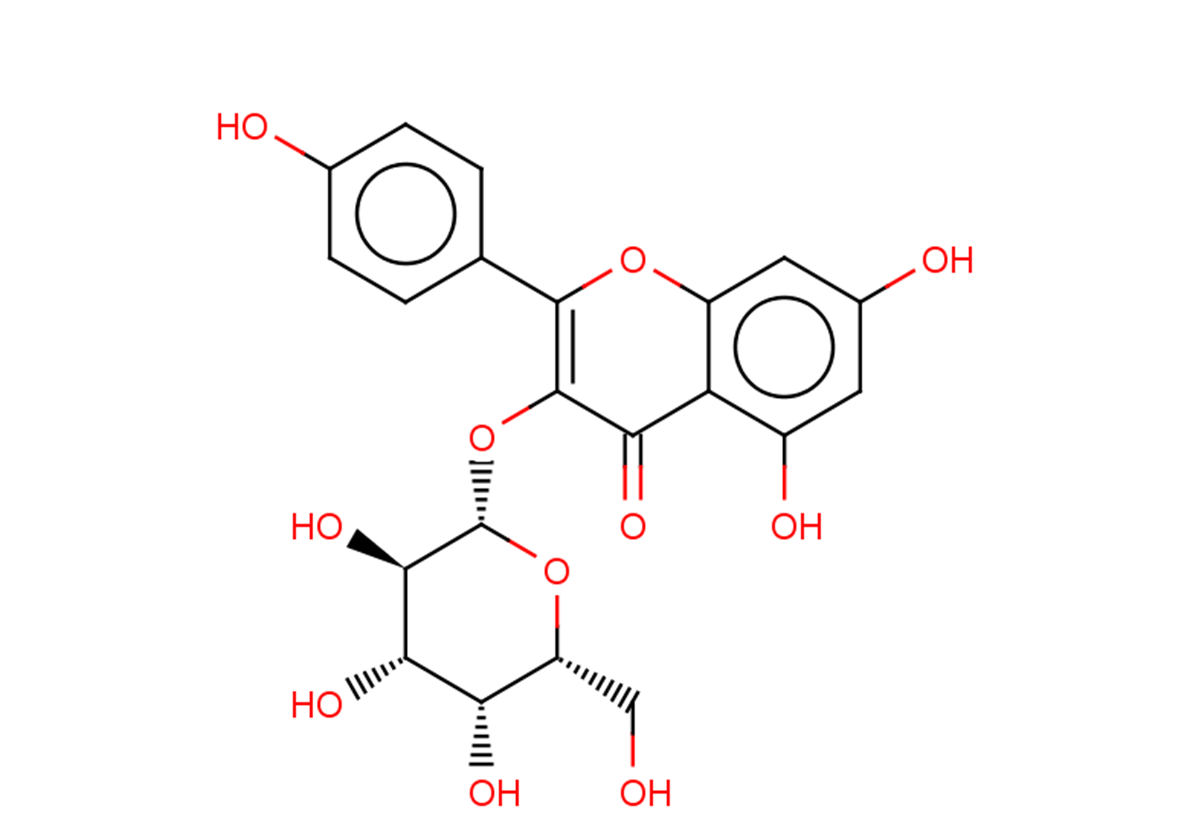
Kaempferol-3-O-galactoside
CAS No. 23627-87-4
Kaempferol-3-O-galactoside( Kaempferol 3-O-beta-D-galactoside )
Catalog No. M24073 CAS No. 23627-87-4
Kaempferol-3-O-galactoside has antioxidant, anti-inflammatory and antinociceptive activities.
Purity : >98% (HPLC)
 COA
COA
 Datasheet
Datasheet
 HNMR
HNMR
 HPLC
HPLC
 MSDS
MSDS
 Handing Instructions
Handing Instructions
| Size | Price / USD | Stock | Quantity |
| 5MG | 312 | In Stock |


|
| 10MG | 500 | In Stock |


|
| 25MG | 791 | In Stock |


|
| 50MG | 1062 | In Stock |


|
| 100MG | Get Quote | In Stock |


|
| 200MG | Get Quote | In Stock |


|
| 500MG | Get Quote | In Stock |


|
| 1G | Get Quote | In Stock |


|
Biological Information
-
Product NameKaempferol-3-O-galactoside
-
NoteResearch use only, not for human use.
-
Brief DescriptionKaempferol-3-O-galactoside has antioxidant, anti-inflammatory and antinociceptive activities.
-
DescriptionKaempferol-3-O-galactoside has antioxidant, anti-inflammatory and antinociceptive activities.
-
In Vitro——
-
In Vivo——
-
SynonymsKaempferol 3-O-beta-D-galactoside
-
PathwayOthers
-
TargetOther Targets
-
RecptorImmunology & Inflammation related
-
Research Area——
-
Indication——
Chemical Information
-
CAS Number23627-87-4
-
Formula Weight448.38
-
Molecular FormulaC21H20O11
-
Purity>98% (HPLC)
-
Solubility——
-
SMILESOC[C@H]([C@@H]([C@@H]([C@H]1O)O)O)O[C@H]1OC1=C(c(cc2)ccc2O)Oc(cc(cc2O)O)c2C1=O
-
Chemical Name——
Shipping & Storage Information
-
Storage(-20℃)
-
ShippingWith Ice Pack
-
Stability≥ 2 years
Reference
1.da Silva I, et al. Structure determination of monohydrated trifolin (kaempferol 3-O-β-D-galactopyranoside) from laboratory powder diffraction data. J Pharm Sci. 2011;100(4):1588-1593.
molnova catalog



related products
-
PATULIN
Patulin is a mycotoxin produced by a variety of molds commonly found in rotting apples including Aspergillus and Penicillium.
-
Marmin
Marmin have anti-ulcer effects, which are ascribed primarily to the maintenance of the mucosal barrier integrity and inhibition of gastric motor activity and secondarily due to the prevention of the effects of endogenous acetylcholine and histamine.
-
Synapsin I - derived...
Synapsin I - derived peptide



 Cart
Cart
 sales@molnova.com
sales@molnova.com


