Indeglitazar
CAS No. 835619-41-5
Indeglitazar( PPM 204 )
Catalog No. M26716 CAS No. 835619-41-5
Indeglitazar is orally available pan-agonist of PPAR (PPAR subtypes alpha (α), delta (δ), and gamma (γ)).
Purity : >98% (HPLC)
 COA
COA
 Datasheet
Datasheet
 HNMR
HNMR
 HPLC
HPLC
 MSDS
MSDS
 Handing Instructions
Handing Instructions
| Size | Price / USD | Stock | Quantity |
| 5MG | 58 | Get Quote |


|
| 10MG | 72 | Get Quote |


|
| 25MG | 147 | Get Quote |


|
| 50MG | 222 | Get Quote |


|
| 100MG | 335 | Get Quote |


|
| 200MG | 494 | Get Quote |


|
| 500MG | Get Quote | Get Quote |


|
| 1G | Get Quote | Get Quote |


|
Biological Information
-
Product NameIndeglitazar
-
NoteResearch use only, not for human use.
-
Brief DescriptionIndeglitazar is orally available pan-agonist of PPAR (PPAR subtypes alpha (α), delta (δ), and gamma (γ)).
-
DescriptionIndeglitazar is orally available pan-agonist of PPAR (PPAR subtypes alpha (α), delta (δ), and gamma (γ)).(In Vitro):Measuring in part functional insulin sensitization capability of the cells, Indeglitazar shows an EC50 of 0.32 μM compared with Rosiglitazone, which shows an EC50 of 13 nM, in an assay of preadipocyte differentiation.(In Vivo):In Zucker rat model of diabetes, Indeglitazar (10 mg/kg; i.v.) significantly lowers the levels of glucose, HbA1C, triglycerides. In the ob/ob model of diabetes and insulin resistance, Indeglitazar significantly decreases glucose, insulin, triglycerides, and free fatty acid levels. The level of Adiponectin (day 21) is essentially unchanged, thus the observed reductions in glucose and HbA1C are achieved in an adiponectin-independent fashion.
-
In VitroIn an assay of preadipocyte differentiation, measuring in part functional insulin sensitization capability of the cells, Indeglitazar shows an EC50 of 0.32 μM compared with Rosiglitazone, which shows an EC50 of 13 nM, although the maximal response obtained from the 2 compounds is comparable.
-
In VivoAn initial assessment of in vivo activity is carried out using the Zucker rat model of diabetes. The significant lowering of glucose, HbA1C, triglycerides, and total cholesterol are observed after i.v. treatment with 10 mg/kg Indeglitazar once per day for 3 weeks. Notably, the level of Adiponectin (on day 21) is essentially unchanged in treated vs. untreated animals (4.8 mcg/mL vs. 4.9 mcg/mL), thus the observed reductions in glucose and HbA1C are achieved in an adiponectin-independent fashion. These differences in the effects of Indeglitazar in vivo may be a consequence of synergy between the 3 PPAR activities or because of the SPPARM profile of the compound, or a combination of these factors. The oral activity of Indeglitazar is assessed in the ob/ob model of diabetes and insulin resistance. Indeglitazar significantly decreases glucose, insulin, triglycerides, and free fatty acid levels.
-
SynonymsPPM 204
-
PathwayMetabolic Enzyme/Protease
-
TargetPPAR
-
RecptorABCG2| Antibacterial| Antibiotic| BCRP
-
Research Area——
-
Indication——
Chemical Information
-
CAS Number835619-41-5
-
Formula Weight389.42
-
Molecular FormulaC19H19NO6S
-
Purity>98% (HPLC)
-
SolubilityIn Vitro:?DMSO : 100 mg/mL (256.79 mM)
-
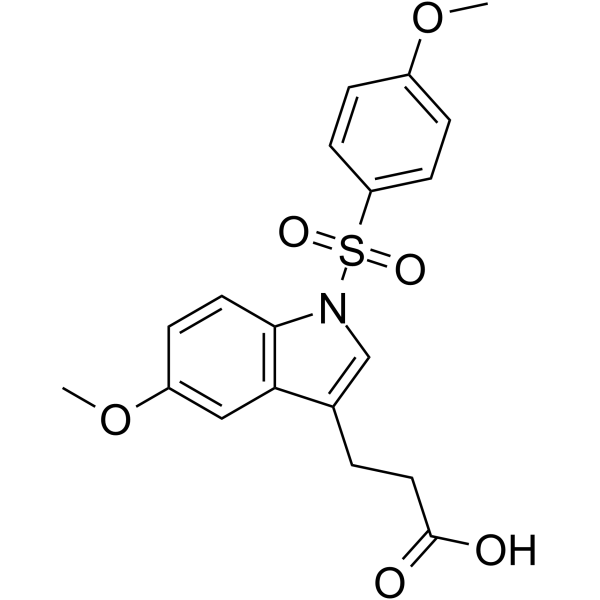
SMILESO=C(O)CCC1=CN(C=2C=CC(OC)=CC21)S(=O)(=O)C3=CC=C(OC)C=C3
-
Chemical Name——
Shipping & Storage Information
-
Storage(-20℃)
-
ShippingWith Ice Pack
-
Stability≥ 2 years
Reference
1.Rabindran SK, et al. Fumitremorgin C reverses multidrug resistance in cells transfected with the breast cancer resistance protein. Cancer Res. 2000 Jan 1;60(1):47-50.
molnova catalog



related products
-
Reglitazar
Reglitazar (JTT-501) is a dual PPARα and PPARγ agonist that is used to study diabetes.
-
BADGE
Bisphenol a diglycidyl ether is a Standardized Chemical Allergen. The physiologic effect of bisphenol a diglycidyl ether is by means of Increased Histamine Release, and Cell-mediated Immunity.
-
MA-0204
MA-0204 is a highly selective and orally available peroxisome proliferator-activated receptor δ (PPARδ) modulator.



 Cart
Cart
 sales@molnova.com
sales@molnova.com


