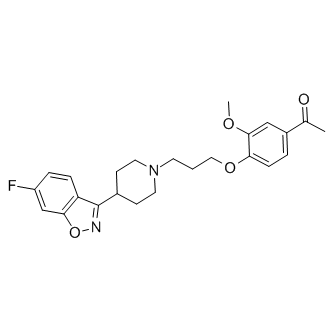
Iloperidone
CAS No. 133454-47-4
Iloperidone( HP-873 )
Catalog No. M11335 CAS No. 133454-47-4
An atypical antipsychotic that exhibits high affinity to serotonin 5HT2A (Ki =5.6 nM), dopamine D2 (Ki=6.3 nM) and D3 (Ki=7.1 nM) and noradrenaline α1 receptors (Ki=0.36 nM).
Purity : >98% (HPLC)
 COA
COA
 Datasheet
Datasheet
 HNMR
HNMR
 HPLC
HPLC
 MSDS
MSDS
 Handing Instructions
Handing Instructions
| Size | Price / USD | Stock | Quantity |
| 5MG | 30 | In Stock |


|
| 10MG | 45 | In Stock |


|
| 25MG | 86 | In Stock |


|
| 50MG | 149 | In Stock |


|
| 100MG | 221 | In Stock |


|
| 200MG | 327 | In Stock |


|
| 500MG | 529 | In Stock |


|
| 1G | Get Quote | In Stock |


|
Biological Information
-
Product NameIloperidone
-
NoteResearch use only, not for human use.
-
Brief DescriptionAn atypical antipsychotic that exhibits high affinity to serotonin 5HT2A (Ki =5.6 nM), dopamine D2 (Ki=6.3 nM) and D3 (Ki=7.1 nM) and noradrenaline α1 receptors (Ki=0.36 nM).
-
DescriptionAn atypical antipsychotic that exhibits high affinity to serotonin 5HT2A (Ki =5.6 nM), dopamine D2 (Ki=6.3 nM) and D3 (Ki=7.1 nM) and noradrenaline α1 receptors (Ki=0.36 nM), moderate affinity for D4 (25 nM), 5HT6 (43 nM), 5HT7 (22 nM), and low affinity for 5HT1A (168 nM), D1 and histamine H1 receptors; is used for the treatment of schizophrenia.Schizophrenia Approved(In Vitro):Iloperidone displays higher affinity for the dopamine D3 receptor (Ki=7.1 nM) than for the dopamine D4 receptor (Ki=25 nM). Iloperidone displays high affinity for the 5-HT6 and 5-HT7 receptors (Ki=42.7 and 21.6 nM, respectively), and is found to have higher affinity for the 5-HT2A (Ki=5.6 nM) than for the 5-HT2C receptor (Ki=42.8 nM).(In Vivo):Iloperidone is eliminated slowly, with a mean t1/2 of 13.5 to 14.0 hours. Coadministration with food did not significantly affect AUC, tmax, or Cmax. These results indicate that the rate of iloperidone's absorption is decreased, but the overall bioavailability is unchanged, when the drug is taken with food. Orthostatic hypotension, dizziness, and somnolence were the most commonly reported adverse events.
-
In VitroIloperidone displays higher affinity for the dopamine D3 receptor (Ki=7.1 nM) than for the dopamine D4 receptor (Ki=25 nM). Iloperidone displays high affinity for the 5-HT6 and 5-HT7 receptors (Ki=42.7 and 21.6 nM, respectively), and is found to have higher affinity for the 5-HT2A (Ki=5.6 nM) than for the 5-HT2C receptor (Ki=42.8 nM).
-
In VivoIloperidone is eliminated slowly, with a mean t1/2 of 13.5 to 14.0 hours. Coadministration with food did not significantly affect AUC, tmax, or Cmax. These results indicate that the rate of iloperidone's absorption is decreased, but the overall bioavailability is unchanged, when the drug is taken with food. Orthostatic hypotension, dizziness, and somnolence were the most commonly reported adverse events.
-
SynonymsHP-873
-
PathwayGPCR/G Protein
-
Target5-HT Receptor
-
RecptorDopamine|α-adrenergicreceptor
-
Research AreaNeurological Disease
-
IndicationSchizophrenia
Chemical Information
-
CAS Number133454-47-4
-
Formula Weight426.4806
-
Molecular FormulaC24H27FN2O4
-
Purity>98% (HPLC)
-
Solubility10 mM in DMSO
-
SMILESCC(C1=CC=C(OCCCN2CCC(C3=NOC4=CC(F)=CC=C34)CC2)C(OC)=C1)=O
-
Chemical NameEthanone, 1-[4-[3-[4-(6-fluoro-1,2-benzisoxazol-3-yl)-1-piperidinyl]propoxy]-3-methoxyphenyl]-
Shipping & Storage Information
-
Storage(-20℃)
-
ShippingWith Ice Pack
-
Stability≥ 2 years
Reference
1. Barr AM, et al. Neuropharmacology. 2006 Sep;51(3):457-65.
2. Szewczak MR, et al. J Pharmacol Exp Ther. 1995 Sep;274(3):1404-13.
3. Sainati SM, et al. J Clin Pharmacol. 1995 Jul;35(7):713-20.
molnova catalog



related products
-
EMD-281014 hydrochlo...
EMD-281014 hydrochloride ((Pruvanserin, LSN2411347, LY-2422347)) is a potent, highly selective 5-HT2A antagonist with Ki of 0.87 nM.
-
Org-13011 fumarate
Org-13011 fumarate1 is an agonist of the 5-HT1A receptor and can be used to study neurological disorders.
-
Ricasetron
Ricasetron (Brl 46470) is a 5-HT3 receptor antagonist with pro-axonal lysis and antiemetic properties, and is used in the study of anxiety and anxiety disorders.



 Cart
Cart
 sales@molnova.com
sales@molnova.com


