HMN-176
CAS No. 173529-10-7
HMN-176( HMN176 | HMN 176 | HMN-176 )
Catalog No. M17369 CAS No. 173529-10-7
HMN-176 is a stilbene derivative which inhibits mitosis, interfering with polo-like kinase-1 (plk1).
Purity : >98% (HPLC)
 COA
COA
 Datasheet
Datasheet
 HNMR
HNMR
 HPLC
HPLC
 MSDS
MSDS
 Handing Instructions
Handing Instructions
| Size | Price / USD | Stock | Quantity |
| 2MG | 46 | In Stock |


|
| 5MG | 72 | In Stock |


|
| 10MG | 110 | In Stock |


|
| 25MG | 178 | In Stock |


|
| 50MG | 332 | In Stock |


|
| 100MG | 494 | In Stock |


|
| 500MG | 1071 | In Stock |


|
| 1G | Get Quote | In Stock |


|
Biological Information
-
Product NameHMN-176
-
NoteResearch use only, not for human use.
-
Brief DescriptionHMN-176 is a stilbene derivative which inhibits mitosis, interfering with polo-like kinase-1 (plk1).
-
DescriptionHMN-176 is an active metabolite of the synthetic antitumor compound HMN-214. HMN-176 shows potent cytotoxicity toward various human tumor cell lines, and in mitotic cells, it causes cell cycle arrest at M phase through the destruction of spindle polar bodies, followed by the induction of DNA fragmentation. However, no direct interactions of HMN-176 with tubulin are observed. Moreover, in animal models, it was observed that oral administration of the prodrug HMN-214 caused no significant nerve toxicity, a severe side effect often associated with microtubule binding agents such as Taxol and VCR.3 In Phase I clinical trials, HMN-214 has caused sensory neuropathy and ileus in some patients. However, the grade and frequency of these adverse effects were much lower than those of typical microtubule binding agents. As expected from the mechanism of action of HMN-214 (induction of G2-M arrest in dividing cells), the main adverse effect was neutropenia.
-
In VitroHMN-176 (2.5 μM) greatly increases the duration of mitosis in hTERT-RPE1 and CFPAC-1 Cell lines. The effect of HMN-176 on spindle morphology does not appear to be related to effects on microtubule polymerization. HMN-176 (2.5, 0.25, and 0.025 μM) inhibits aster formation in a concentration dependent manner. HMN-176 (0.1, 1.0, or 10.0 μg/mL) demonstrates inhibitory effects in multiple tumors, with notable activity seen in breast, nonsmall-cell lung, and ovarian cancer specimens. HMN-176 demonstrates activity towards 63% of the breast (5/8), 67% of the non-small cell lung (4/6), and 57% of the ovarian (4/7) tumor specimens treated with 10.0 μg/mL. HMN-176 shows potent cytotoxicity, with a mean IC50 value of 118 nM. HMN-176 displays similar cytotoxicity against tumors with various characteristics from different organs. Treatment with 3 μM HMN-176 suppresses the expression of MDR1 mRNA by 56%. HMN-176 has no significant effect on the residual promoter activity.
-
In VivoAfter p.o. of HMN-214 to male rats, the prodrug is not detected in the plasma, while plasma levels of HMN-176 peaks at 2 h and gradually decreases thereafter.
-
SynonymsHMN176 | HMN 176 | HMN-176
-
PathwayOthers
-
TargetOther Targets
-
RecptorOthers
-
Research AreaCancer
-
Indication——
Chemical Information
-
CAS Number173529-10-7
-
Formula Weight382.43
-
Molecular FormulaC20H18N2O4S
-
Purity>98% (HPLC)
-
SolubilityIn Vitro:?DMSO : 30 mg/mL (78.45 mM)
-
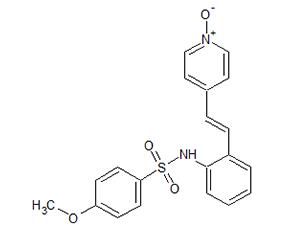
SMILESO=S(=O)(c1ccc(OC)cc1)Nc1ccccc1/C=C/c1cc[n+](cc1)[O-]
-
Chemical Name(E)-4-(2-(4-methoxyphenylsulfonamido)styryl)pyridine 1-oxide
Shipping & Storage Information
-
Storage(-20℃)
-
ShippingWith Ice Pack
-
Stability≥ 2 years
Reference
1. DiMaio MA, et al. Mol Y Ther. 2009 Mar;8(3):592-601.
molnova catalog



related products
-
EGF Receptor Substra...
EGF Receptor Substrate 2 (Phospho-Tyr5) is a biologically active peptide derived from an autophosphorylation site (Tyr992) of EGFR.
-
Tubulin polymerizati...
Tubulin polymerization-IN-55 is a potent Tubulin Polymerization inhibitor with potential anti-angiogenic and anti-tumor activity, demonstrating anti-proliferative effects against A549, K562, HepG2, MDA-MB-231, and HFL-1.
-
Di-O-methylhonokiol
Di-O-methylhonokiol (Honokiol dimethyl ether), a phenolic component of Magnolia grandiflora L, exhibits antimicrobial activity and significant antioxidant activity against Gram-positive and acid-resistant bacteria and fungi.



 Cart
Cart
 sales@molnova.com
sales@molnova.com


