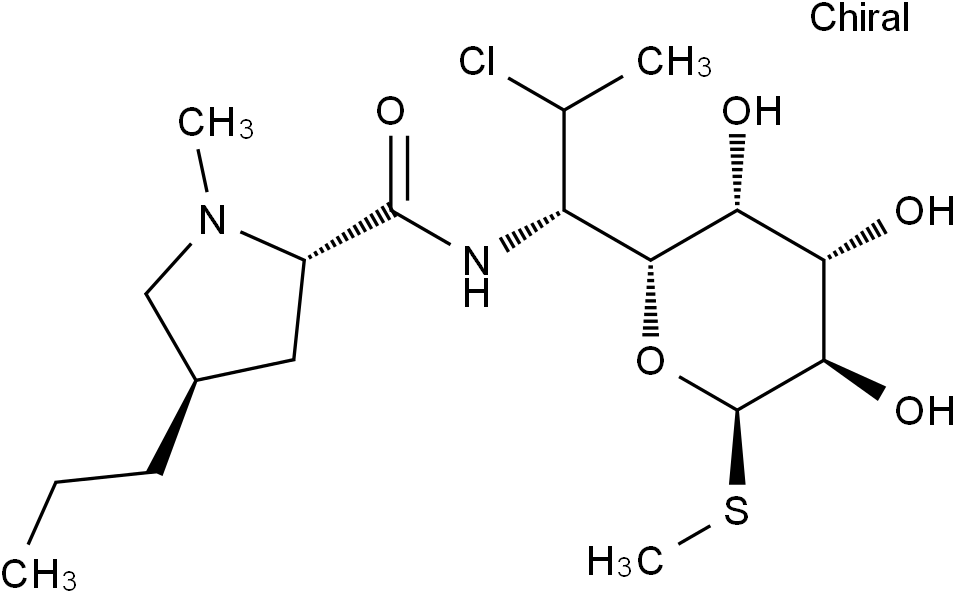
Clindamycin
CAS No. 18323-44-9
Clindamycin( U 21251 )
Catalog No. M12825 CAS No. 18323-44-9
Clindamycin inhibits protein synthesis by acting on the 50S ribosomal.
Purity : >98% (HPLC)
 COA
COA
 Datasheet
Datasheet
 HNMR
HNMR
 HPLC
HPLC
 MSDS
MSDS
 Handing Instructions
Handing Instructions
| Size | Price / USD | Stock | Quantity |
| 25MG | 38 | In Stock |


|
| 50MG | 53 | In Stock |


|
| 100MG | 73 | In Stock |


|
| 200MG | Get Quote | In Stock |


|
| 500MG | Get Quote | In Stock |


|
| 1G | Get Quote | In Stock |


|
Biological Information
-
Product NameClindamycin
-
NoteResearch use only, not for human use.
-
Brief DescriptionClindamycin inhibits protein synthesis by acting on the 50S ribosomal.
-
DescriptionClindamycin inhibits protein synthesis by acting on the 50S ribosomal.
-
In VitroCell Viability Assay Cell Line:THP-1 cells with stimulated E. coli O55:B5 Concentration:3.13-50 μg/mL Incubation Time:1-18 h Result:Reduced TNF-α concentrations after pretreatment for 4 or 18 h at 25 and 50 μg/mL.Cell Viability Assay Cell Line:Osteoblast cell culture model Concentration:0-500 μg/mL Incubation Time:24-72 h Result:Increased alkaline phosphatase (ALP)-activity at 10 μg/mL at 24 and 48 h.Increased LDH values at 500 μg/mL at 24 and 48 h, indicating cytotoxicity.Increased calcification at 10 and 25 μg/mL,decreased or no calcification was found at 50 μg/ml.
-
In Vivo——
-
SynonymsU 21251
-
PathwayOthers
-
TargetOther Targets
-
RecptorOthers
-
Research AreaOther Indications
-
Indication——
Chemical Information
-
CAS Number18323-44-9
-
Formula Weight424.98
-
Molecular FormulaC18H33ClN2O5S
-
Purity>98% (HPLC)
-
SolubilityDMSO:85 mg/mL (200.0 mM); Ethanol:85 mg/mL (200.0 mM); Water:<1 mg/mL (<1 mM)
-
SMILESCCC[C@@H]1C[C@@H](C(N[C@@H]([C@H]2O[C@@H]([C@@H]([C@H]([C@H]2O)O)O)SC)[C@@H](Cl)C)=O)N(C1)C
-
Chemical Name(2S,4R)-N-((1S,2S)-2-chloro-1-((2R,3R,4S,5R,6R)-3,4,5-trihydroxy-6-(methylthio)tetrahydro-2H-pyran-2-yl)propyl)-1-methyl-4-propylpyrrolidine-2-carboxamide
Shipping & Storage Information
-
Storage(-20℃)
-
ShippingWith Ice Pack
-
Stability≥ 2 years
Reference
1. Keusch GT, et al. J Infect Dis, 1976, 133(5), 578-587.
molnova catalog



related products
-
PF-05221304
PF-05221304 is an orally bioavailable, liver-targeted inhibitor of acetyl-CoA carboxylase (ACC), an enzyme that catalyzes the first committed step in de novo lipogenesis (DNL).
-
MID-1
MID-1 is an inhibitor of MG53-IRS-1 (Mitsugumin 53-Insulin Receptor Substrate-1) interaction.?It disrupts molecular association of MG53 with IRS-1 and abolishes MG53-induced IRS-1 ubiquitination and degradation in skeletal muscle, leading to elevated IRS-1 expression level and increased insulin signaling and glucose uptake.
-
Pinocembrin 7-O-beta...
Pinocembrin 7-O-beta-D-glucoside is a natual product isolated from?Penthorum chinense Pursh.



 Cart
Cart
 sales@molnova.com
sales@molnova.com


