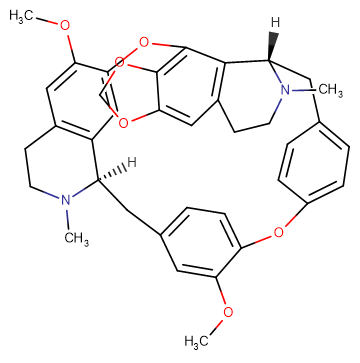
Cepharanthine
CAS No. 481-49-2
Cepharanthine( O-Methylcepharanoline | NSC 623442 )
Catalog No. M14609 CAS No. 481-49-2
Cepharanthine is a biscoclaurine alkaloid inhibiting tumor necrosis factor (TNF)-α-mediated NFκB stimulation, plasma membrane lipid peroxidation and platelet aggregation and suppressing cytokine production.
Purity : >98% (HPLC)
 COA
COA
 Datasheet
Datasheet
 HNMR
HNMR
 HPLC
HPLC
 MSDS
MSDS
 Handing Instructions
Handing Instructions
| Size | Price / USD | Stock | Quantity |
| 50MG | 41 | In Stock |


|
| 100MG | 52 | In Stock |


|
| 200MG | Get Quote | In Stock |


|
| 500MG | Get Quote | In Stock |


|
| 1G | Get Quote | In Stock |


|
Biological Information
-
Product NameCepharanthine
-
NoteResearch use only, not for human use.
-
Brief DescriptionCepharanthine is a biscoclaurine alkaloid inhibiting tumor necrosis factor (TNF)-α-mediated NFκB stimulation, plasma membrane lipid peroxidation and platelet aggregation and suppressing cytokine production.
-
DescriptionCepharanthine is a biscoclaurine alkaloid inhibiting tumor necrosis factor (TNF)-α-mediated NFκB stimulation, plasma membrane lipid peroxidation and platelet aggregation and suppressing cytokine production.
-
In VitroApoptosis Analysis Cell Line:MDAMB-231 and BT549 cells Concentration:2 μM Incubation Time:48 h Result:Cepharanthine alone minimally increased apoptosis (~5% to ~10%), whereas combinated with Epirubicin (HY-13624) markedly increased apoptosis (~50%).Western Blot AnalysisCell Line:MDAMB-231 cells Concentration:2 μM Incubation Time:48 h Result:Combinated with Epirubicin (HY-13624) markedly resulted in oxidation of the actin-remodeling protein cofilin, which promoted formation of an intramolecular disulfide bridge between Cys39, Cys80 and Ser3 dephosphorylation, leading to mitochondria translocation of cofilin. Combinated with Epirubicin (HY-13624) induced mitochondrial fission in MDA-MB-231 cells. Immunofluorescence Cell Line:K562 cells or MIA-PaCa-2 cells Concentration:10,20,25,50 μM Incubation Time:0.5 h or 1 h Result:Made the intracellular localization of Doxorubicin (HY-15142A) in cytoplasmic vesicles shifted to the nucleoplasm. Decreased red AO (weakly basic fluorescence probe) fluorescence by dose-dependent mannar in K562 cells. Cell Viability Assay Cell Line:P. falciparum cultivated in type A+ human erythrocytes Concentration:2 μM Incubation Time:48 h Result:Blocked P. falciparum development in ring stage with IC50s of 3.059, 0.927, 2.276, and 1.803 μM for FCM29, W2, 3D7 and K1, respectively.
-
In VivoAnimal Model:MDA-MB-231 cell xenografts in miceDosage:12 mg/kg Administration:Intraperitoneal injection (i.p.), once daily for 36 days Result:Combinated with Epirubicin (HY-13624) greatly enhanced the therapeutic efficacy compared with administration of either drug alone.Animal Model:LPS-induced pulmonary vascular injury in male Wistar rats Dosage:10 mg/kg Administration:Intraperitoneal injection (i.p.), single dose Result:Decreased LPS-induced pulmonary vascular injury. Significantly inhibited the increases in plasma tumor necrosis factor-a (TNF-a) concentrations. Animal Model:LPS-induced Wistar rat model of systemic inflammation Dosage:10 mg/kg Administration:Intraperitoneal injection (i.p.), single dose Result:Significantly inhibited the increase in LPS-induced IL-6, TNF-α and nitrate/nitrite levels. Reduced interstitial edema and inflammatory cell compared with the control group. Reduced pathologic abnormalities, such as vacuolization, dot necrosis, striped necrosis, and bridging necrosis appeared, and inflammatory cells compared with the control group.group compared with the LPS group. Animal Model:Mice pain models in Kunming (KM) strain male and female mice Dosage:10 mg/kg Administration:Intraperitoneal injection (i.p.)Result:Resulted in a dose-dependent antinociceptive effect with an ED50 value of 24.5 mg/kg in mice pain models.Significantly decreased the intestinal propulsion with maximal inhibition at 33.6%.
-
SynonymsO-Methylcepharanoline | NSC 623442
-
PathwayOthers
-
TargetOther Targets
-
RecptorOthers
-
Research AreaCancer
-
Indication——
Chemical Information
-
CAS Number481-49-2
-
Formula Weight606.73
-
Molecular FormulaC37H38N2O6
-
Purity>98% (HPLC)
-
SolubilityDMSO: 100 mg/mL (164.82 mM)
-
SMILESCOC1=CC2=C3C=C1OC4=C(OCO5)C5=CC6=C4[C@@](CC7=CC=C(OC(C(OC)=C8)=CC=C8C[C@@]3([H])N(C)CC2)C=C7)([H])N(C)CC6
-
Chemical Name(15S,31R)-36,53-dimethoxy-16,32-dimethyl-15,16,17,18,31,32,33,34-octahydro-2,6-dioxa-1(4,5)-[1,3]dioxolo[4,5-g]isoquinolina-3(7,1)-isoquinolina-5,7(1,4)-dibenzenacyclooctaphane
Shipping & Storage Information
-
Storage(-20℃)
-
ShippingWith Ice Pack
-
Stability≥ 2 years
Reference
1.Huang H, et al. Inflammation. 2014 Feb;37(1):235-46.
molnova catalog



related products
-
Tlatlancuayin
The herbs of Iresine herbstii.
-
Vinaginsenoside R4
Vinaginsenoside R4 has melanogenic inhibitory activity, it may have potential as a new skin whitening compound.
-
Luteolin-3-O-beta-D-...
Luteolin-3-O-beta-D-glucuronide is active in the inhibition of nitrite production in macrophages.



 Cart
Cart
 sales@molnova.com
sales@molnova.com


